Aromatic aldehyde preparation method employing oxygen to catalyze and oxidize aromatic primary alcohol
A technology for the catalytic oxidation of aromatic primary alcohols, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds. The effect of less dosage, high product yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
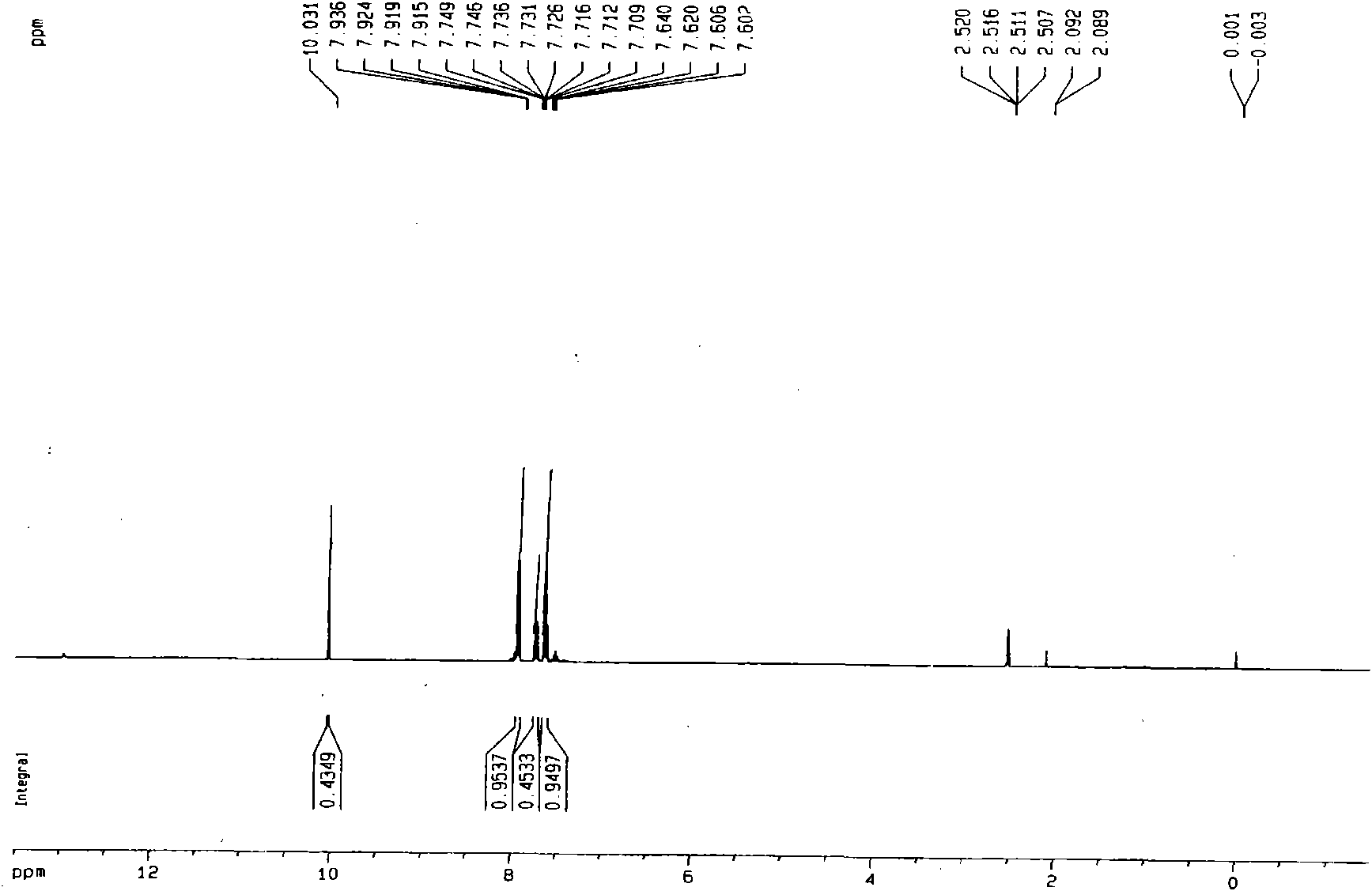
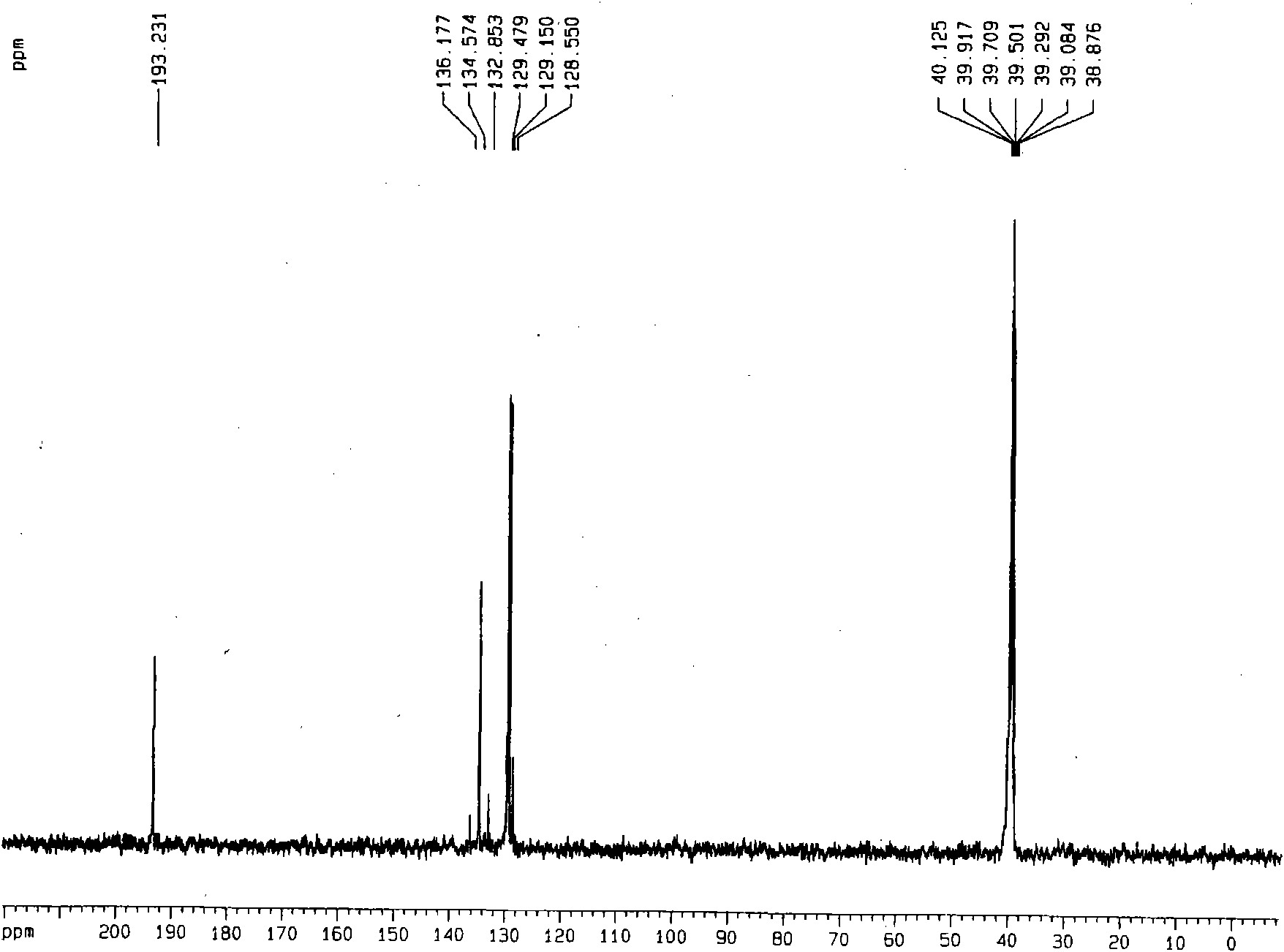
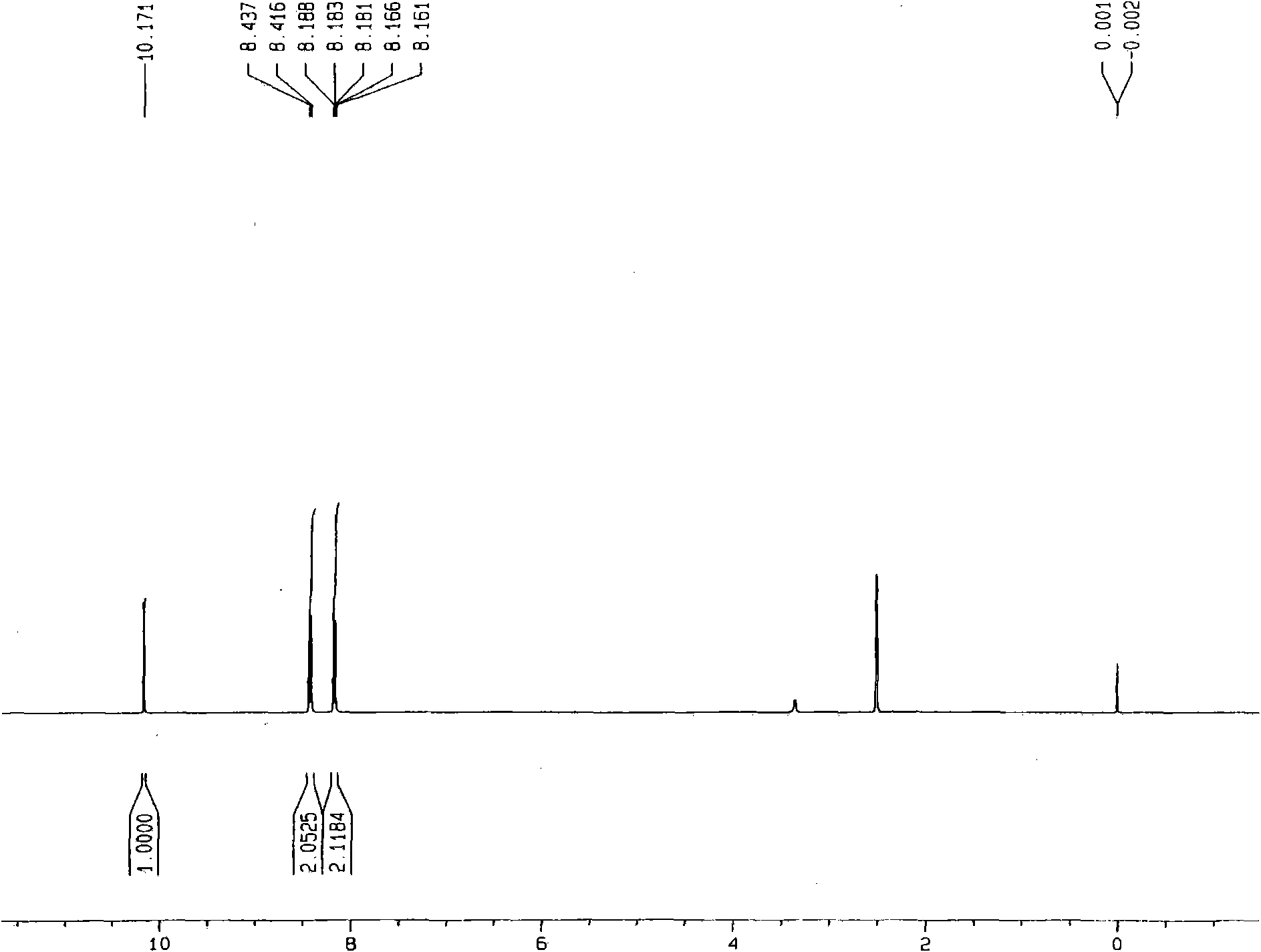
[0020] Example 1: Add 5.4g of benzyl alcohol, 0.427g of vanadyl sulfate, and 0.175g of sodium nitrite into a 500mL reaction kettle, add 50mL of acetonitrile, add 250μL of water, close the kettle, fill it with oxygen at a pressure of 0.5MPa, and raise the temperature under stirring To 80 ℃, and keep 2h, if the partial pressure of oxygen drops, add oxygen. After the reaction, cool to room temperature. Sampling is analyzed by gas chromatography, and the conversion rate of benzyl alcohol is greater than 99%, and the selectivity of benzaldehyde is greater than 98%. The separation yield of benzaldehyde was 93%. through 1 HNMR, 13 CNMR, GC-MS identified benzaldehyde.
Embodiment 2
[0021] Example 2: Add 5.4g of benzyl alcohol, 0.427g of vanadyl sulfate, and 0.175g of sodium nitrite into a 500mL reaction kettle, add 50mL of acetonitrile, close the kettle, fill it with oxygen at a pressure of 0.5MPa, and heat up to 80°C under stirring. And keep 2h, if the oxygen partial pressure drops, add oxygen. After the reaction, cool to room temperature. Sampling was analyzed by gas chromatography, the conversion rate of benzyl alcohol was 11.3%, and the selectivity of benzaldehyde was greater than 98%.
Embodiment 3
[0022] Example 3: 5.4g of benzyl alcohol, 0.427g of vanadyl sulfate, and 0.253g of potassium nitrite were added to a 500mL reaction kettle, 50mL of acetonitrile was added, and 500 μL of water was added, the kettle was closed, and the pressure of oxygen was 0.5MPa, and the temperature was raised under stirring To 50 ℃, and keep 10h, if the oxygen partial pressure drops, add oxygen. After the reaction, cool to room temperature. Sampling is analyzed by gas chromatography, the conversion rate is greater than 99%, and the selectivity is greater than 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com