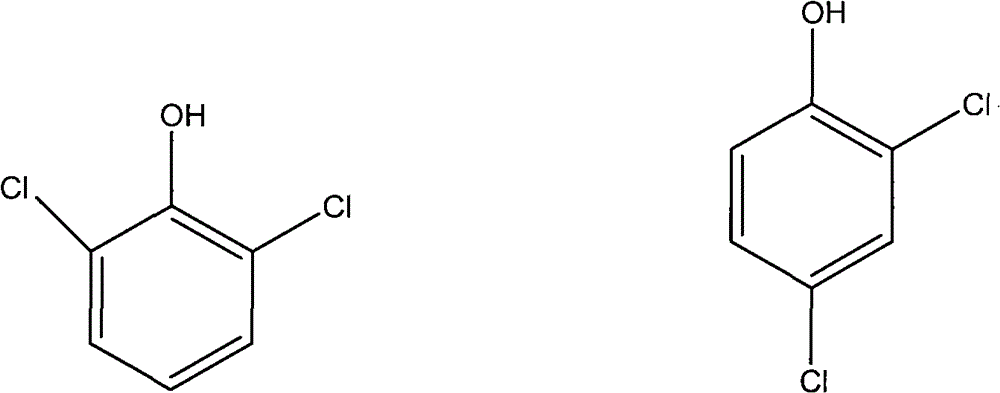Method for separating 2,4-Dichlorophenol and 2,6-Dichlorophenol
A technology of dichlorophenol and mixture, applied in the field of separation 2, can solve the problems of small investment, high equipment requirements, and difficult post-processing, etc., and achieve the effects of industrial scale-up, rapid separation, and low implementation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039]In a 200ml beaker, add 150ml ethanol and 50g 2,4 dichlorophenol, 2,6-dichlorophenol mixture, detect through high performance liquid chromatography (the same below) wherein 2,4-dichlorophenol and 2,6-dichlorophenol The content of phenol was 50% and 50% respectively, then 16g of morpholine was added, stirred and heated at 70° C. for 2 minutes until the solution was clear. Placed under the greenhouse for 7 days, colorless and transparent crystals were precipitated, and filtered under reduced pressure to obtain 31.5 g of filter cake crystals and filtrate. compound, and 2,4-dichlorophenol is left in the filtrate, and then high-purity 2,4-dichlorophenol 22.8g can be obtained by distillation, the yield is 91%, and the purity is 96% (high performance liquid Phase chromatography detection, the same below). Use 100ml of ethanol to recrystallize the crude 2,6-dichlorophenol once, add it to 60ml of 10% dilute hydrochloric acid solution for a full reaction at room temperature, then ...
example 2
[0041] In a 500ml beaker, add 200ml methyl alcohol and 50g 2,4 dichlorophenol, 2,6-dichlorophenol mixture, wherein the content of 2,4-dichlorophenol and 2,6-dichlorophenol is respectively 60% and 40%, then add 16g of triethylenediamine, stir and heat at 60°C for 2 minutes until the solution is clear. Placed under the greenhouse for 6 days, colorless and transparent crystals precipitated, and filtered under reduced pressure to obtain 30.8 g of filter cake crystals and filtrate. The crystals were 2,6-dichlorophenol crude product, which was 2,6-dichlorophenol and triethylene The compound of diamine, while 2,4-dichlorophenol is left in the filtrate, and then 28.0 g of high-purity 2,4-dichlorophenol can be obtained by distillation, the yield is 93%, and the purity is 98%. Recrystallize the crude product of 2,6-dichlorophenol once with 100ml of methanol, add it to 60ml of 10% dilute hydrochloric acid solution for a full reaction at room temperature, then add 60ml of benzene for extr...
example 3
[0043] Add 150ml ethanol and 50g 2,4 dichlorophenol, 2,6-dichlorophenol mixture in 200ml beaker, wherein 2,4-dichlorophenol and 2, the content of 6-dichlorophenol are respectively 60% and 40%, then add 12.5g piperazine, stir and heat at 70°C for 2 minutes until the solution is clear. Placed at room temperature for 8 days, colorless and transparent crystals precipitated, and filtered under reduced pressure to obtain 30.5 g of filter cake crystals and filtrate. The crystals were crude 2,6-dichlorophenol, which was the mixture of 2,6-dichlorophenol and piperazine. The complex, while 2,4-dichlorophenol is left in the filtrate, and then high-purity 2,4-dichlorophenol 29.1g can be obtained by distillation, the yield is 97%, and the purity is 98%. Use 100ml of acetone to recrystallize the crude 2,6-dichlorophenol once, add it to 60ml of 10% dilute hydrochloric acid solution for a full reaction at room temperature, then add 60ml of chloroform for extraction, let it stand for layers, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com