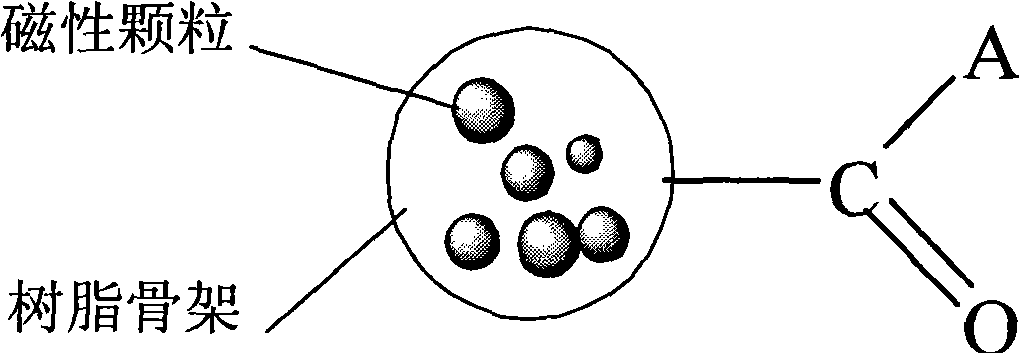
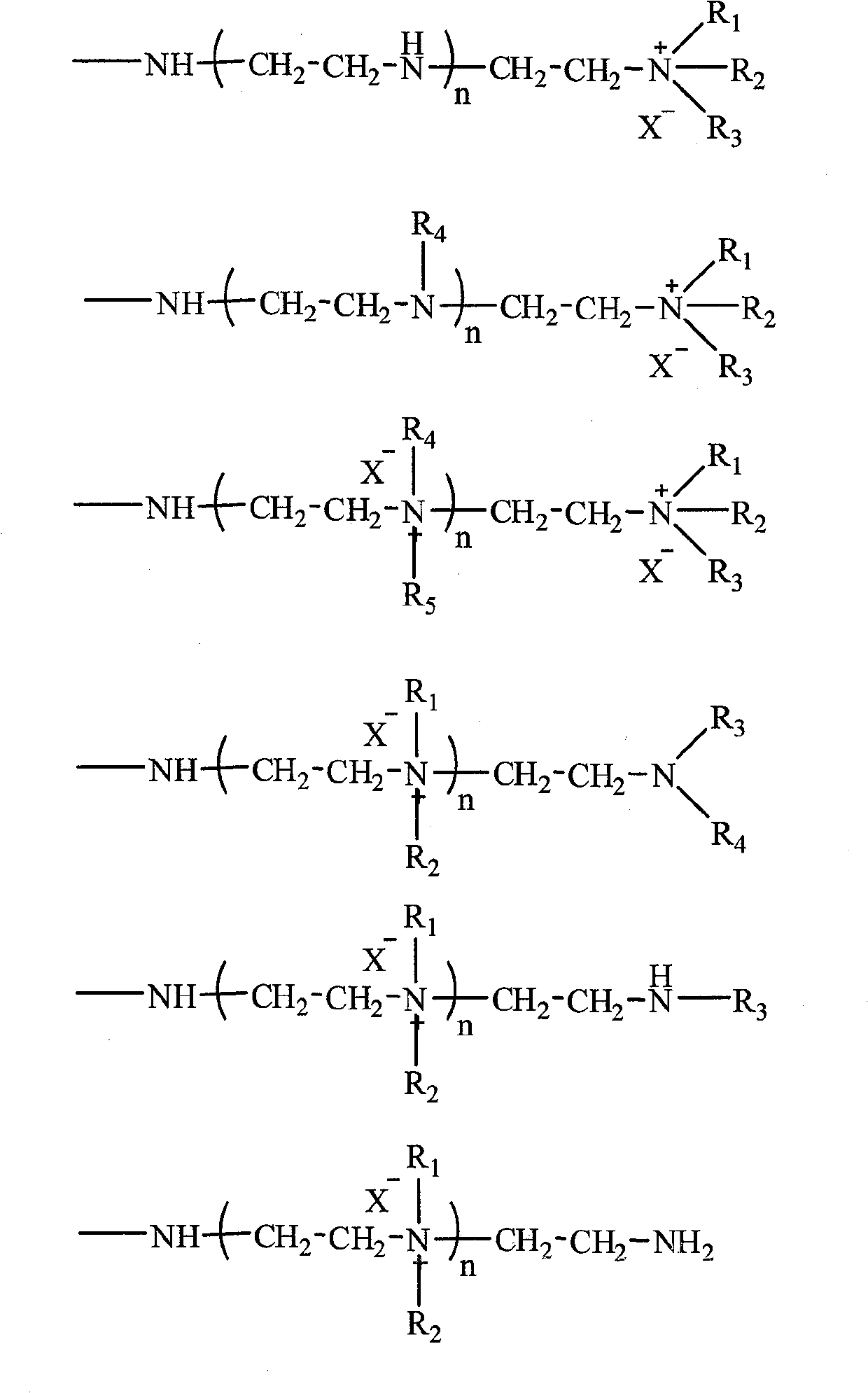
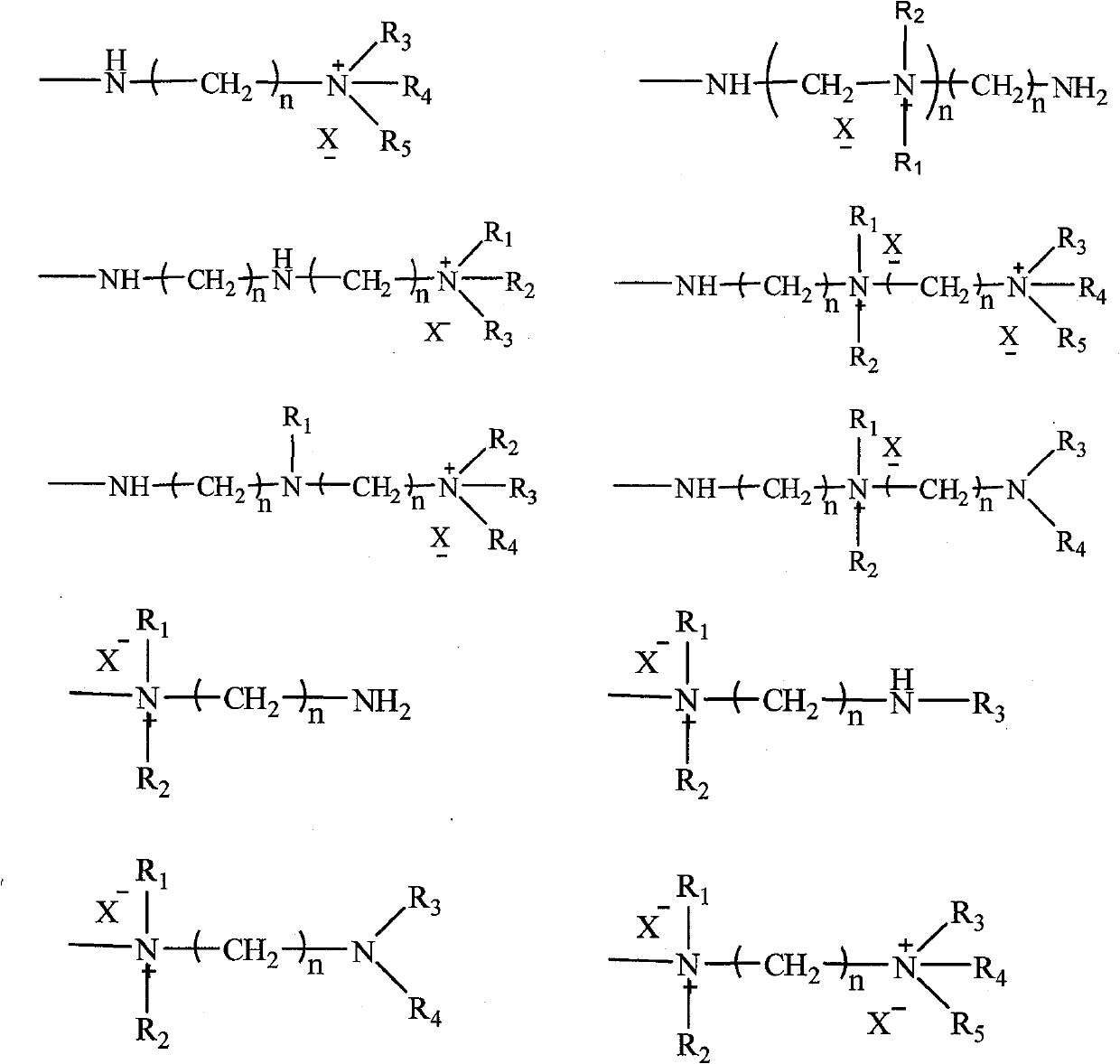
Magnetic acrylic acid series strongly basic anion exchange microballoon resin and preparation method thereof
A technology of acrylic acid and anion, which is applied in the field of acrylic strong base anion exchange microsphere resin and its preparation. It can solve the problems of difficult control of the synthesis process, uneven particle size of the resin, and difficulty in uniform dispersion of magnetic particles, and achieve excellent adsorption and high strength. Effects of alkali exchange capacity and desorption kinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 500 g of an aqueous solution containing 3% by weight of gelatin and 23% of sodium chloride was added to a 2L three-necked flask, and the stirring speed was controlled at 100-150 rpm. A mixed solution of 100g methyl acrylate, 2g trimethacrylate (trimethylolpropyl) ester, 1.0g azobisisobutyronitrile, 100g toluene, 53g ethyl acetate and 102g tetrafluoroethylene with a particle size of about 0.1μm were prepared. After the ferric oxide magnetic particles are uniformly mixed, put into a three-necked flask and heat up to 50 ° C, hold for 8 hours, heat up to 85 ° C, hold for 15 hours and then discharge; after washing with ethanol and drying, add 2 times the amount of resin The weight of ethylenediamine was kept at 100 ° C for 8 hours and then discharged; after cleaning, 300 mL of 10% liquid caustic soda was added, 40.8 g of methyl iodide was added, and the material was discharged after reacting at 20 ° C for 10 hours. Strong base ion exchange microsphere resin.
[0037] The ob...
Embodiment 2
[0040]500 g of an aqueous solution containing polyvinyl alcohol with a weight ratio of 0.2% and 5% of sodium chloride was added to a 2L three-necked flask, and the stirring speed was controlled at 150-200 rpm. 90g methyl acrylate, 10g methacrylic acid, 15g trimethacrylate (trimethylolpropyl), 5g triallyl cyanurate, 0.48g benzoyl peroxide, 10g toluene, 2g 200# The mixed solution of solvent oil and 6g of ferric oxide magnetic particles with a particle size of about 0.1 μm were uniformly mixed, then added to a three-necked flask and heated to 55 ° C, kept for 8 hours, heated to 85 ° C, and discharged after 10 hours . After washing and drying with ethanol, N,N-dimethylpropanediamine, which is 2.5 times the weight of the resin, was added and kept at 150° C. for 30 hours before discharging. After cleaning, 300 mL of 10% liquid caustic soda was added, 63.0 g of methyl iodide was added, and the mixture was reacted at 40° C. for 2 hours and then discharged. The magnetic strong alkali...
Embodiment 3
[0044] 500 g of active calcium phosphate and 20% sodium chloride aqueous solution with a weight ratio of 3% were added to a 2L three-necked flask, and the stirring speed was controlled at 400-500 rpm. 90g methyl acrylate, 10g butyl methacrylate, 5g trimethacrylate (trimethylolpropyl), 5g divinylbenzene, 0.4g azobisisobutyronitrile, 0.4g benzoyl peroxide, 22g 200 # The mixed solution of solvent oil was uniformly mixed with 11 g of ferroferric oxide magnetic particles with a particle size of about 0.1 μm, then added to a three-necked flask and heated to 60°C for 5 hours, then heated to 88°C and kept for 12 hours. After washing and drying with ethanol, N,N-dimethylbutanediamine, which is 4 times the weight of the resin, was added and kept at 200° C. for 10 hours before discharging. After cleaning, 300 mL of 10% liquid caustic soda was added, 120 g of methyl iodide was added, and the mixture was reacted at 50° C. for 20 hours and then discharged. The magnetic strong alkali ion e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com