Method for preparing benzaldehyde by oxidizing toluene
A technology for benzaldehyde and toluene, applied in the field of toluene oxidation to prepare benzaldehyde, can solve the problems of small market of benzyl alcohol acetate, low conversion rate of single-stage reactor, uneconomical industrial production, etc. Separation, less waste liquid and less residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
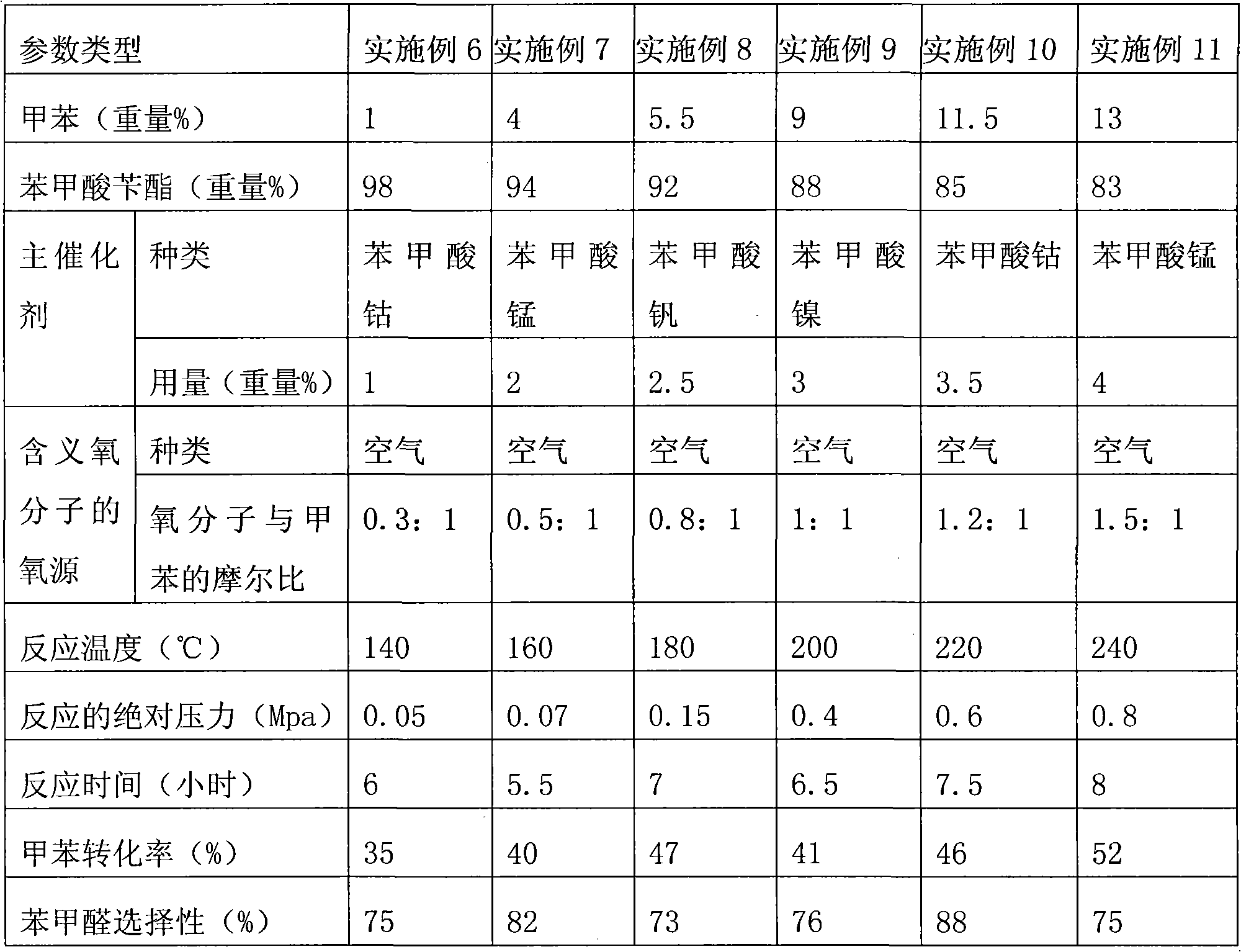
[0024] The method for preparing benzaldehyde by oxidation of toluene in this example is as follows. In a 500ml reactor, add 240g of solvent benzyl benzoate, 60g of toluene and 3.0g of catalyst cobalt benzoate, turn on the stirring, and let air into the reactor so that the absolute pressure in the reactor is 0.3MPa, then turn on the heating to heat the reaction liquid to 180-185℃, the air flow rate is 0.4L / min, and the absolute pressure of the reactor is maintained at 0.35MPa. The water produced by the reaction azeotropes with toluene, and is condensed with oil and water After separation, toluene is returned to the reactor, and water is treated as waste liquid. After reacting for 6 hours, the air cooling was turned off. Quantitative analysis by gas chromatography showed that the reaction solution contained 28.6 g of toluene, 28.3 g of benzaldehyde, 3.2 g of benzyl alcohol and 4.2 g of benzoic acid. The conversion rate of toluene was 52.3%, and the selectivity of benzaldehyde was...
Embodiment 2
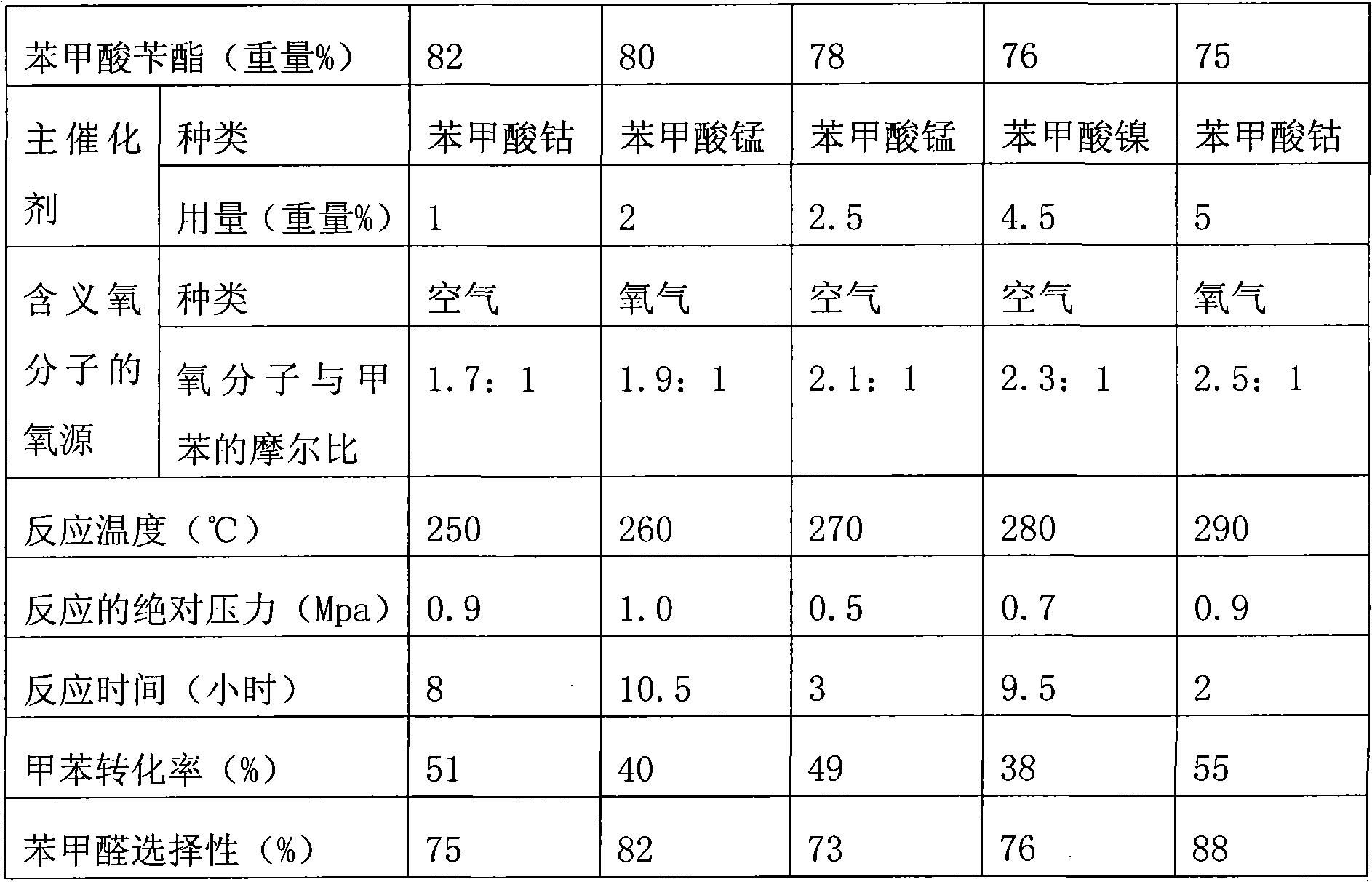
[0026] Add 280g of solvent benzyl benzoate, 20g of toluene, 3.0g of cobalt benzoate, and 1.0g of manganese benzoate into a 500ml reactor, turn on the stirring, and react under normal pressure. The flow of air is 0.2L / min, and the reaction is heated. The temperature is 225-230℃, and the mixed liquid of benzyl benzoate, catalyst and toluene is added at a flow rate of 30g / h. The content of toluene in the mixed liquid is 30%. The reaction liquid is continuously released to keep the liquid level in the reactor at a high level. The reaction liquid is distilled to separate the benzaldehyde, benzyl alcohol and benzoic acid. The remaining benzyl benzoate and the catalyst mother liquor are purified and then mixed with 30% of the raw material toluene, and the flow rate is 30g / h. In the reactor; stop adding fresh benzyl benzoate, catalyst and toluene mixture at the same time. The gas phase on the reaction liquid contains toluene, benzaldehyde and water. The toluene, benzaldehyde and water ...
Embodiment 3
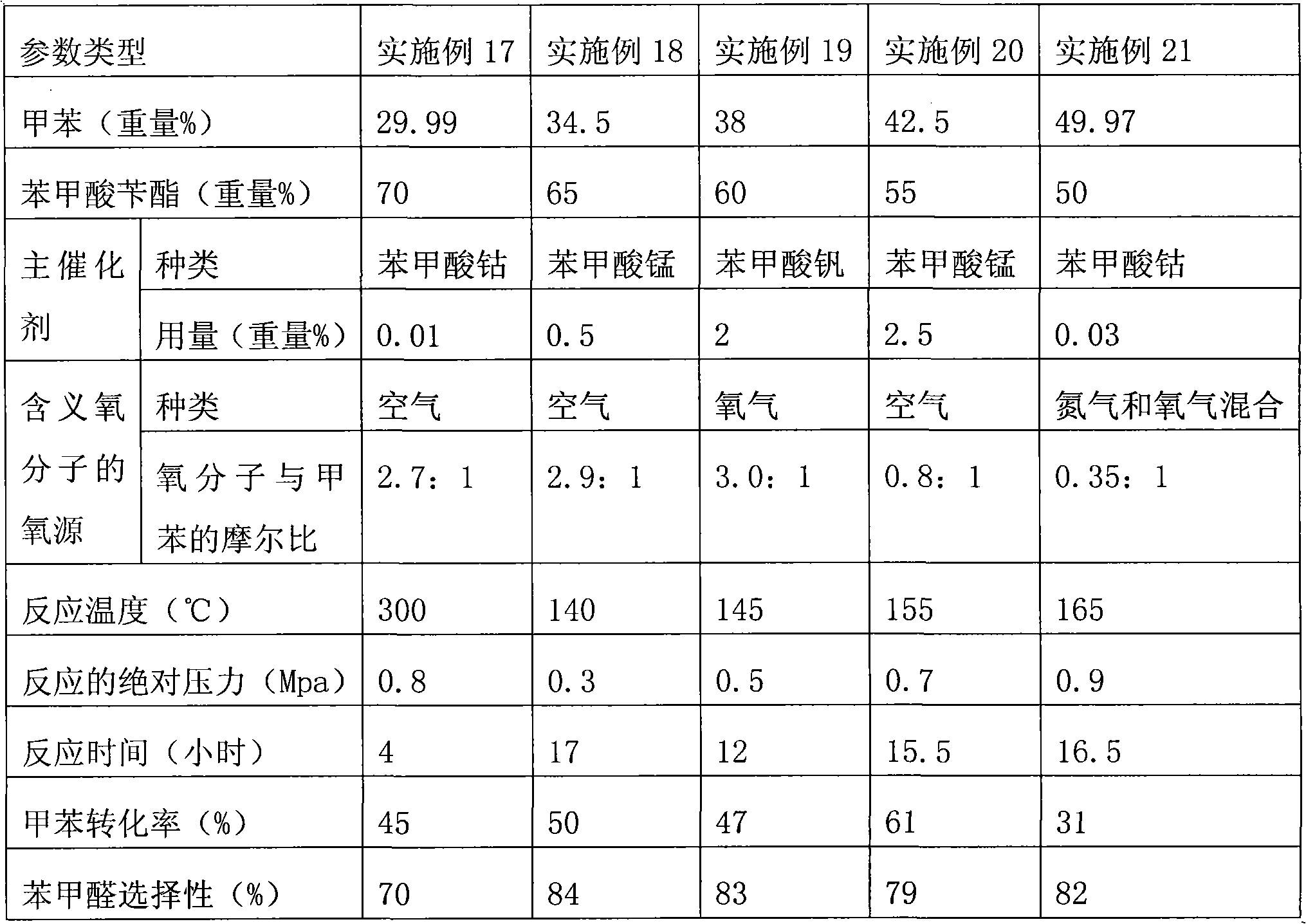
[0028] Add 500g of solvent benzyl benzoate, 40g of toluene, 6.0g of cobalt benzoate, 1.0g of manganese benzoate, and 0.5g of nickel benzoate into an 800ml bubble column reactor, and react under normal pressure with a flow of air of 0.2L / min, heating to a reaction temperature of 220-225°C, adding a mixture of benzyl benzoate, catalyst and toluene at a flow rate of 30 g / h. The toluene content of the mixture is 30%. The reaction solution is continuously released to make the reactor liquid Keep the position at the initial height, distill the released reaction liquid to separate benzaldehyde, benzyl alcohol and benzoic acid, and the remaining benzyl benzoate and the catalyst mother liquor are purified and mixed with 30% raw material toluene and mixed with a flow rate of 30g / h Return to the reactor; stop adding fresh benzyl benzoate, catalyst and toluene mixture. The gaseous phase on the reaction liquid contains toluene, benzaldehyde and water. The gaseous phase containing toluene, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com