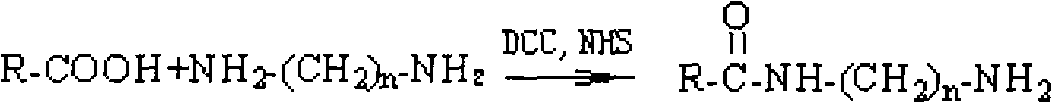Preparation and application of amphiphilic polysaccharide conjugate and medicinal compositions thereof
An amphiphilic polysaccharide and conjugate technology, which is applied in the directions of drug combination, drug delivery, and non-active ingredients medical preparations, etc., can solve the problem that the bridging group connecting the drug and the polymer is not easy to find, and the dose of the drug is insufficient. To achieve the anti-cancer effect, the treatment effect is not optimal and other problems, to achieve excellent biodegradability, excellent biocompatibility, and the effect of avoiding capture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
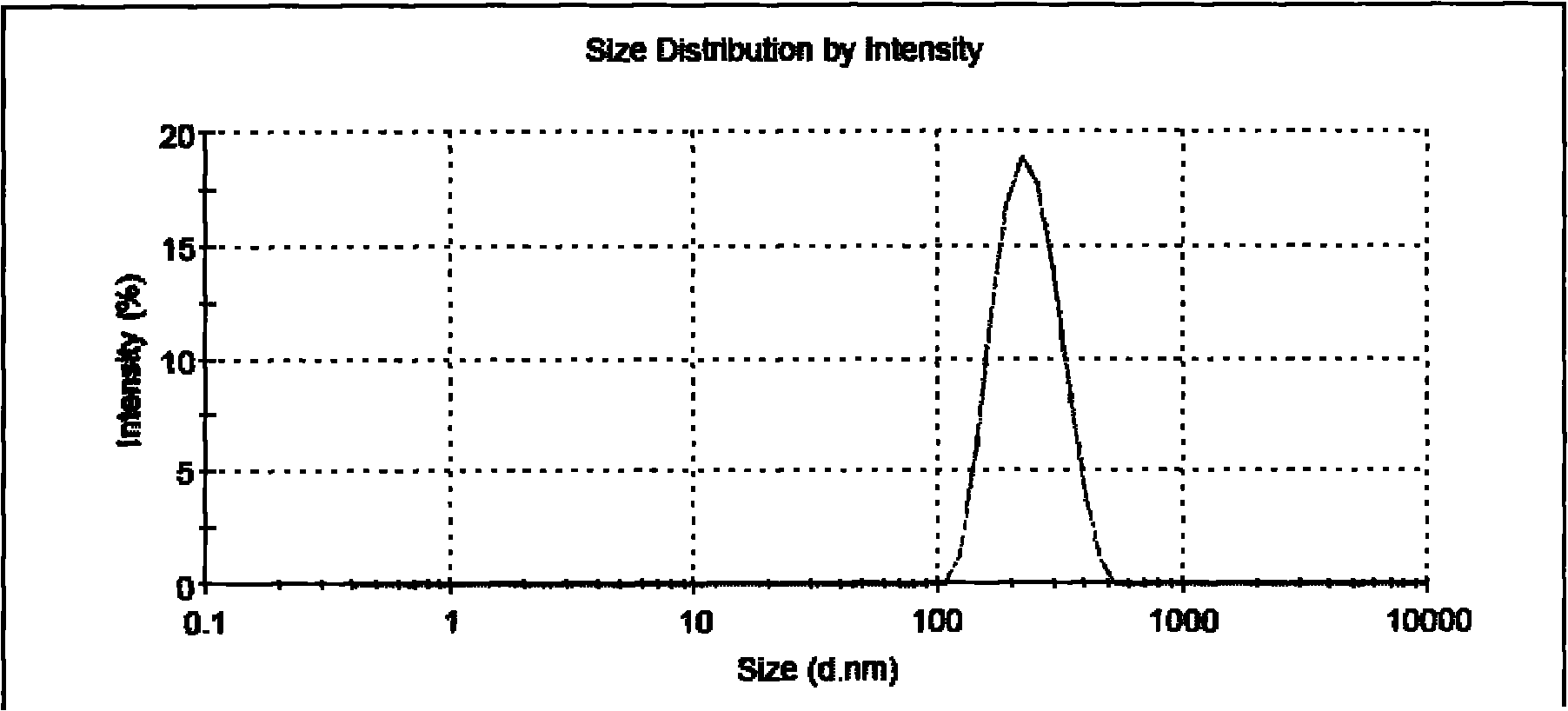
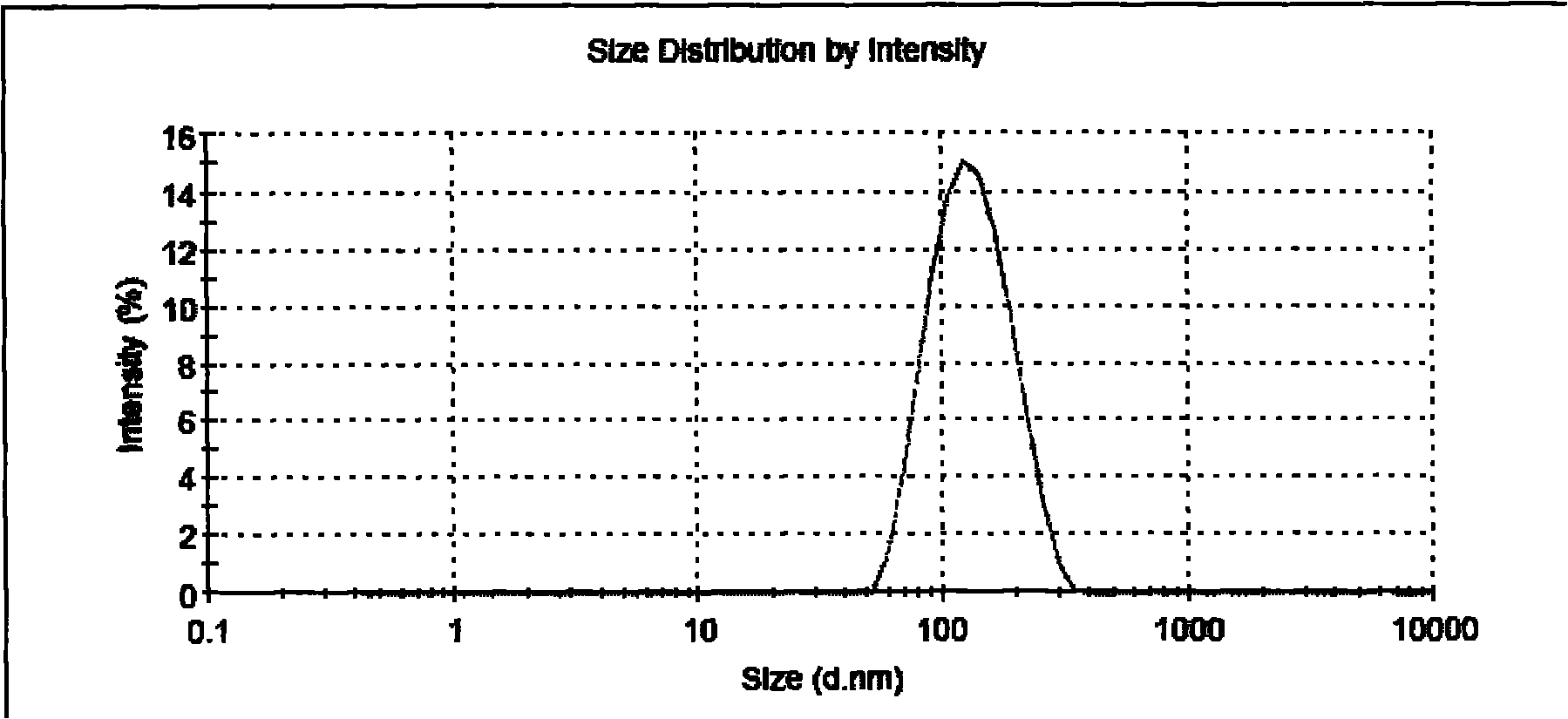
Image
Examples
Embodiment 1
[0054] Embodiment 1: the synthesis of heparin-all-trans retinoic acid
[0055] Take 10mmol all-trans retinoic acid, 12mmol dicyclohexylcarbodiimide (DCC), 15mmol hydroxysuccinimide (NHS), dissolve in 30ml N,N-dimethylformamide, protect from light and nitrogen , reacted in ice bath for 30min, and then rose to room temperature for 24h. After the reaction, the precipitate was filtered off, and a large amount of ethyl acetate was added to wash the precipitate. The filtrate was extracted, the ethyl acetate layers were combined, and the solvent was removed by rotary evaporation to obtain the activated intermediate ester of all-trans retinoic acid. Dissolve 1mmol of all-trans retinoic acid activated intermediate ester in 10ml of dichloromethane, slowly drop into 3mmol / ml of ethylenediamine in dichloromethane solution under ice-bath conditions, and monitor the reaction by thin layer chromatography (TLC method) After completion, the reaction liquid is extracted, the organic layers ar...
Embodiment 2
[0056] Embodiment 2: the synthesis of chondroitin sulfate-all-trans retinoic acid
[0057] Take 10mmol of all-trans retinoic acid, 12mmol of dicyclohexylcarbodiimide (DCC), and 15mmol of hydroxysuccinimide (NHS), dissolve them in 30ml of N,N-dimethylformamide, protect from light and nitrogen, The reaction was carried out in an ice bath for 30 min, and then raised to room temperature for 24 h. After the reaction, the precipitate was filtered off, and a large amount of ethyl acetate was added to wash the precipitate. The filtrate was extracted, the ethyl acetate layers were combined, and the solvent was removed by rotary evaporation to obtain the activated intermediate ester of all-trans retinoic acid. Dissolve 1mmol of all-trans retinoic acid activated intermediate ester in 10ml of dichloromethane, slowly drop into 3mmol / ml of ethylenediamine in dichloromethane solution under ice-bath conditions, and monitor the reaction by thin layer chromatography (TLC method) After complet...
Embodiment 3
[0058] Example 3: Synthesis of Heparin-Methotrexate
[0059] Take 10mmol of methotrexate, 15mmol of dicyclohexylcarbodiimide (DCC), and 15mmol of hydroxysuccinimide (NHS), dissolve them in 20ml of N,N-dimethylformamide, keep away from light, under the protection of nitrogen, and store on ice Bath reaction 30min, then rise to room temperature reaction 24h. After the reaction, the precipitate was filtered off, and a large amount of ethyl acetate was added to wash the precipitate. The filtrate was extracted, the ethyl acetate layers were combined, and the solvent was removed by rotary evaporation to obtain the activated intermediate ester of methotrexate. Dissolve 1mmol of methotrexate activated intermediate ester in 20ml of dichloromethane, slowly drop into 1.5mmol / ml of ethylenediamine in dichloromethane solution under ice-bath conditions, and monitor the reaction by thin layer chromatography (TLC method) After completion, the reaction solution is extracted, the organic layer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com