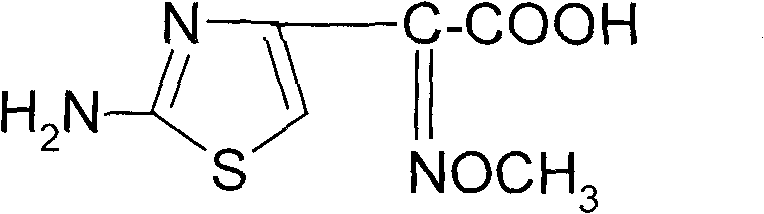
Synthetic method of 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid
A technique for the synthesis of aminothiaxamic acid, which is applied in the direction of organic chemistry, can solve the problems of adverse effects on the health of operators and environmental safety, difficult control of the reaction, slow reaction speed, etc., and achieves easy control of process operation, shortened production cycle, and safe and convenient use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] ①. Homogeneous oximation reaction: preparation of ethyl 2-hydroxime acetoacetate
[0044] Add 60mL of ethanol aqueous solution (volume ratio 1:2), 20mL of ethyl acetoacetate, and 10g of sodium nitrite into the three-necked flask, stir, and add 30g of glacial acetic acid dropwise at a temperature of 0-5°C for 1 hour. After the addition, continue The reaction was stirred for 2 h, extracted with 25 mL of chloroform, separated, and the organic phase was directly used for the next reaction.
[0045] ②.Methylation reaction: Preparation of ethyl 2-methoxyiminoacetoacetate
[0046] Transfer the organic phase of ethyl 2-hydroxime acetoacetate prepared in step ① into a flask, and add 110 mL of 20% sodium carbonate solution and 0.4 g of polyethylene glycol. Then, start to add 19 mL of dimethyl sulfate dropwise, control the reaction temperature at 10°C to 14°C, and the pH value at 8 to 10, complete the dropwise addition in 1 hour, and keep warm for 3 hours after the addition. Chl...
Embodiment 2
[0056] ①. Homogeneous oximation reaction: preparation of ethyl 2-hydroxime acetoacetate
[0057] Add 60mL of ethanol aqueous solution (volume ratio 1:2), 20mL of ethyl acetoacetate, and 10g of sodium nitrite into the three-neck flask, stir, and add 40g of glacial acetic acid dropwise at a temperature of 0-5°C, and finish adding in 1 hour. The reaction was stirred for 2 h, extracted with 25 mL of chloroform, separated, and the organic phase was directly used for the next reaction.
[0058] ②.Methylation reaction: Preparation of ethyl 2-methoxyiminoacetoacetate
[0059] Transfer the organic phase of ethyl 2-hydroxime acetoacetate prepared in step ① into a flask, add 110 mL of 20% sodium carbonate solution and 0.4 g of tetrabutylammonium bromide. Then, start to add 19 mL of dimethyl sulfate dropwise, control the reaction temperature at 10°C to 14°C, and the pH value at 8 to 10, complete the dropwise addition in 1 hour, and keep warm for 3 hours after the addition. Chloroform 20...
Embodiment 3
[0069] ①. Homogeneous oximation reaction: preparation of ethyl 2-hydroxime acetoacetate
[0070] Add 60mL of ethanol aqueous solution (volume ratio 1:2), 20mL of ethyl acetoacetate, and 10g of sodium nitrite into the three-neck flask, stir, and add 50g of glacial acetic acid dropwise at a temperature of 0-5°C for 1 hour. After the addition, continue The reaction was stirred for 2 h, extracted with 25 mL of chloroform, separated, and the organic phase was directly used for the next reaction.
[0071] ②.Methylation reaction: Preparation of ethyl 2-methoxyiminoacetoacetate
[0072] Transfer the organic phase of ethyl 2-hydroxime acetoacetate prepared in step ① into a flask, add 110 mL of 20% sodium carbonate solution and 0.5 g of benzyltrimethylammonium chloride. Then, start to add 19 mL of dimethyl sulfate dropwise, control the reaction temperature at 10°C to 14°C, and the pH value at 8 to 10, complete the dropwise addition in 1 hour, and keep warm for 3 hours after the additio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com