Application of aporphine alkaloid, oxoaporphine alkaloid and derivatives thereof in preparing antibacterial medicines
A technology of alkaloids and derivatives, applied in antibacterial drugs, medical preparations containing active ingredients, pharmaceutical formulations, etc., to achieve the effects of convenient use, significant antibacterial activity, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Extraction and separation of monomeric alkaloids 1 to 7 from the root tubers of the small leaflet
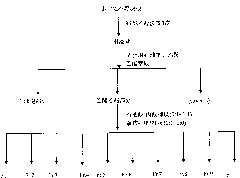
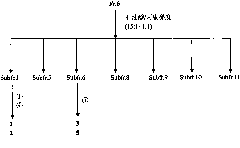
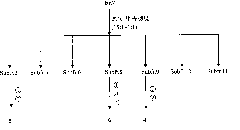
[0046] Stephania succifera H.S.Lo et Tsoong (Stephania succifera H.S.Lo et Tsoong) dried root tuber 16.0Kg was pulverized and then leached three times with ethanol with a volume fraction of 95% at room temperature, each time for 7 days. The ethanol was recovered under reduced pressure until there was no alcohol smell, and the ethanol extract was obtained. It is dispersed in water to form a suspension, such as figure 1 As shown, extract 3 times with petroleum ether and ethyl acetate successively, respectively concentrate to obtain petroleum ether extract (168.5g) and ethyl acetate extract (90.3g), after the remaining water part is filtered, pass through D-101 Pore adsorption resin column chromatography, eluting with water and methanol in sequence, collecting the methanol eluate, and concentrating under reduced pressure to obtain methanol extract (133.0 g). Et...
Embodiment 2
[0047] Example 2: Physicochemical and spectral data of monomeric compounds
[0048] Dehydrocbanine (1): Pale yellow needle-like crystals (chloroform), mp 155-156°C, yellow in the presence of sulfuric acid with a volume fraction of 10%, and orange-red in the reaction with potassium bismuth iodide. EI-MS m / z: 337[M] + , combined with 1 H-NMR and 13 C-NMR spectrum data deduces that its molecular formula is C 20 h 19 NO 4 . 1 H-MR (400MHz, CDCl 3 )δ: 8.66 (1H, d, J=9.1Hz, H-11), 7.03 (1H, d, J=9.1Hz, H-10), 6.90 (1H, s, H-3), 6.87 (1H, s, H-7), 6.18 (2H, s, OCH 2 O), 3.99 (3H, s, 8-OCH 3 ), 3.98 (3H, s, 9-OCH 3 ), 3.36 (2H, t, J=6.0Hz, 5-CH 2 ), 3.20 (2H, t, J=6.0Hz, 4-CH 2 ), 3.12 (3H, s, N-CH 3 ); 13 C-NMR (100MHz, CDCl 3 )δ: 143.7(C-1), 117.4(C-1a), 118.7(C-1b), 145.2(C-2), 123.3(C-3), 127.3(C-3a), 30.6(C-4 ), 50.5(C-5), 141.6(C-6a), 94.4(C-7), 129.3(C-7a), 157.5(C-8), 150.2(C-9), 106.8(C-10) , 108.8(C-11), 118.2(C-11a), 100.8(OCH 2 O), 40.5(N-CH 3 ), 60.5 (8...
Embodiment 3
[0055] Embodiment 3: the in vitro antibacterial activity of aporphyllum and oxo-aporphyllin alkaloids
[0056] 3.1 Experimental materials
[0057] 3.1.1 Test drug
[0058] Dehydrogrambanine, grambanine, 7-Oxodehydrocaaverine, 7-oxodehydrocaaverine, 4,5-dioxo-dehydrocarbanine, oxidized grambanine, (-)-Sukhodianine: from small leaves The isolated monomeric compounds were extracted.
[0059] Chloroform: Tianjin Fuyu Fine Chemical Co., Ltd., batch number 20080901
[0060] Dehydrogrambanine, grambanine, 7-Oxodehydrocaaverine, 7-oxodehydrocaaverine, 4,5-dioxodehydrocarbanine, oxidized grambanine, (-)-Sukhodianine were prepared with chloroform respectively At a concentration of 1 mg / ml, the test drug was stored at room temperature 25°C. Before the experiment, the drugs were fully mixed, and the pharmacodynamic tests were carried out separately.
[0061] 3.1.2 Experimental strains
[0062] Staphylococcus aureus (Staphylococcus aureus) ATCC 51650 and methicillin-resistant Staphyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com