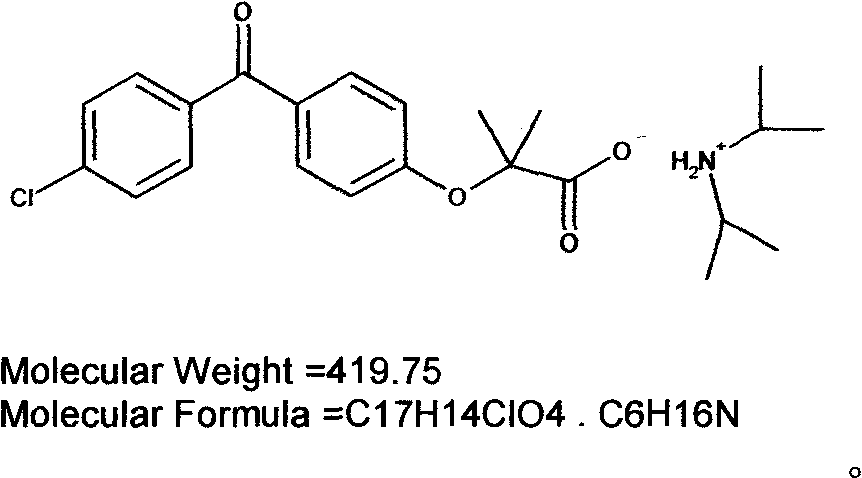
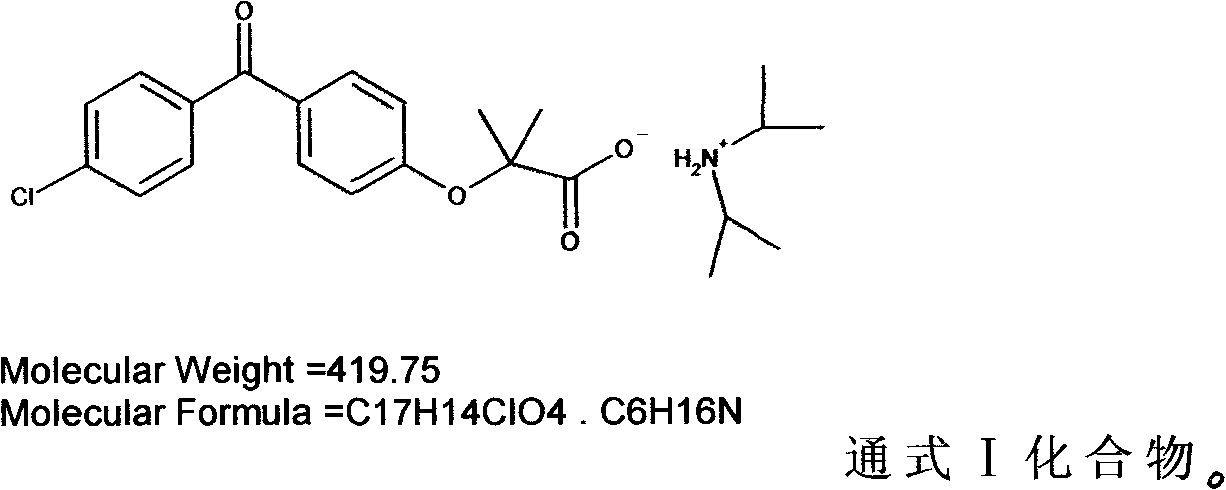
Diisopropylamine fenofibrate, preparation method of same, medicinal composition and use of same
A technology of acid diisopropylamine salt and fenofibrate acid, which is applied in the field of fenofibrate acid diisopropylamine salt and can solve problems such as toxic and side effects, poor water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
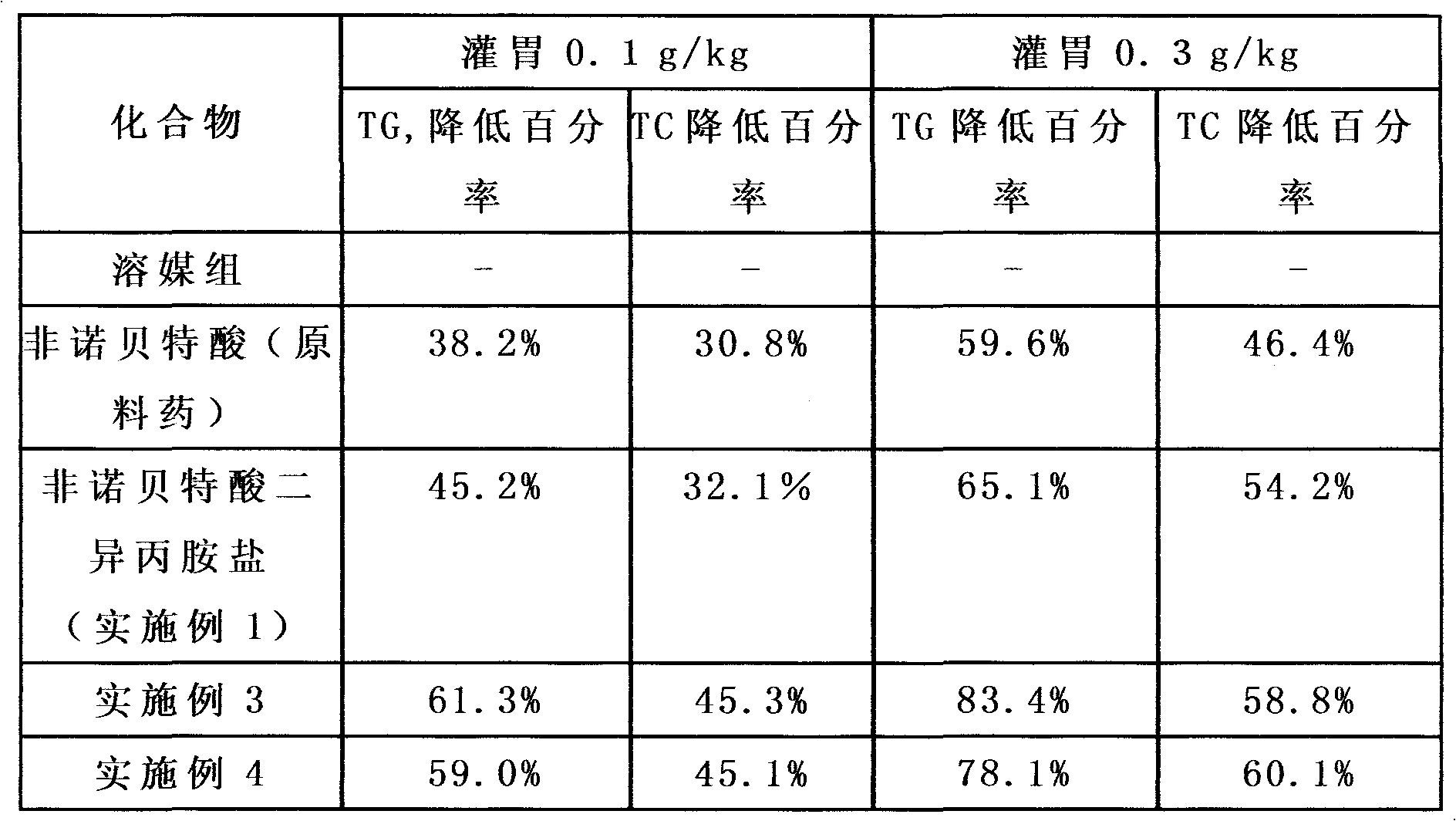
Examples
Embodiment 1
[0035] Preparation of fenofibric acid diisopropylamine salt:
[0036] Dissolve 31.8g of fenofibric acid in 150mL of ether, add 10.1g of diisopropylamine, reflux for 30min, cool to 15°C, a large amount of white solid is formed, suction filtered, the solid is washed once with 30mL of ether, and dried in vacuum (-0.1 MPa, 45°C, 3-5 hours), weighed 30.8g, yield 96.55%. 1HNMR(CDCl 3 )δ: 9.09 (br s, 2H), 7.74-7.76 (dt, 2H), 7.70-7.72 (dt, 2H), 7.44-7.46 (dt, 2H), 6.93-6.95 (dt, 2H), 3.37-3.40 (m, 2H), 1.7 (s, 6H), 1.46-1.48 (d, 12H). MS: 317, 102; elemental analysis, theoretical value: C 65.78%, H 7.20%, Cl 8.44%, N 3.34%; measured value of C 65.76%, H 7.22%, Cl 8.43%, N 3.33%.
Embodiment 2
[0038] Preparation of fenofibrate sodium:
[0039] Suspend 31.8g of fenofibric acid in 200mL of 0.5mol / L sodium hydroxide solution, raise the temperature to 50℃, stir for 30min, until all fenofibric acid is dissolved, add 50mL of ethanol, cool, and precipitate a white solid, water pump Vacuum filtration, vacuum drying (-0.1MPa, 45°C, 3-5 hours), weigh 31.6g, yield 92.75%. 1HNMR(D 2 O) δ: 7.74-7.76 (dt, 2H), 7.70-7.72 (dt, 2H), 7.44-7.46 (dt, 2H), 6.93-6.95 (dt, 2H), 1.7 (s, 6H). MS: 317; elemental analysis, theoretical value: C 59.93%, H 4.14%, Cl 10.40%; measured value of C 59.90%, H 4.16%, Cl 10.43%.
Embodiment 3
[0041] Preparation of fenofibric acid diisopropylamine salt composition tablet
[0042] Take the following raw materials, each serving 100 grams.
[0043] 100 parts of fenofibric acid diisopropylamine salt
[0044] Mannitol 60 parts
[0045] 100 servings of lactose
[0046] 50 parts starch
[0047] Sodium Carboxymethyl Starch 5 parts
[0048] 5 servings of talcum powder
[0049] Povidone 3 parts
[0050] After passing the above-mentioned raw materials through a 100-mesh sieve respectively, they are mixed uniformly according to the equal-volume progressive addition method, and directly compressed into 1000 tablets. The obtained tablet swells rapidly and disintegrates and disperses in water and 0.1 mol / L hydrochloric acid solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com