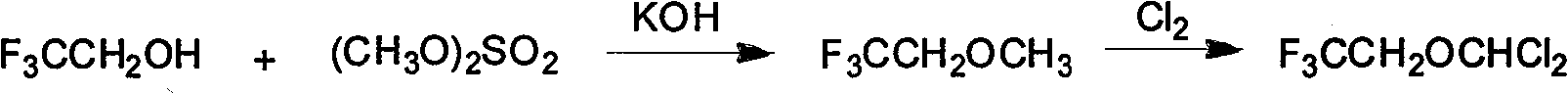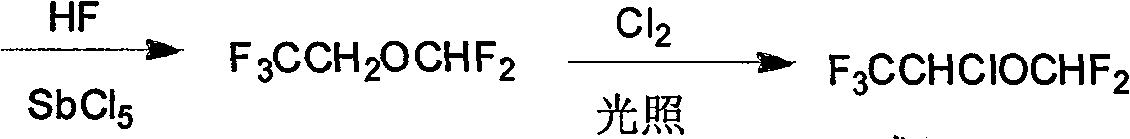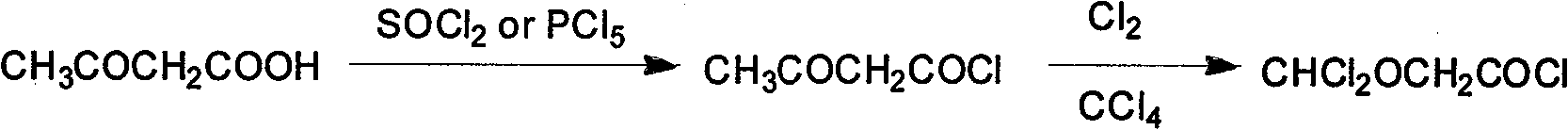Synthesis method of isoflurane
A synthetic method, the technology of isoflurane, which is applied in the field of medicine and chemical industry, can solve the problems of low conversion rate of ether compounds, increase of by-products, low conversion rate, etc., and achieve the effect of improving purity, reducing the generation of by-products, and improving conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Chlorination of (2,2,2-trifluoroethyl)-difluoromethyl ether
[0050] Add 450g of ether compound and 60ml of water into a four-neck flask equipped with an ice-salt condenser, a thermometer, a mechanical stirrer and an air inlet duct, start the stirrer, first pass nitrogen gas for 3-5min, and discharge the oxygen in it, and then Chlorine gas is passed down. The water bath controls the reaction temperature at 5-15° C. When about 40 liters (about 1.8 mol) of chlorine gas is fed in, the chlorine gas flow is stopped, and the stirring reaction is continued for 30 minutes.
[0051] The reaction solution was transferred to a separatory funnel for layering, and the organic layer solution was washed once with ice-cold 10% (w / w) sodium hydroxide aqueous solution, then washed with cold water until neutral, and dried with anhydrous calcium chloride.
[0052] Put the above dried mixed solution into a rectification bottle, install a high-efficiency fractionation column for ...
Embodiment 2
[0053] Example 2: Reduction of (1,1-dichloro-2,2,2-trifluoroethyl)-difluoromethyl ether
[0054] Add 20ml of isopropanol to the residue after rectification in Example 1, control the temperature at 20-30°C, and light for 2 hours under the protection of stirring and nitrogen flow, distill the reactant, and collect the fraction at 60-68°C. Isoflurane and acetone azeotrope 19.6g.
Embodiment 3
[0055] Example 3: Chlorination of (2,2,2-trifluoroethyl)-difluoromethyl ether
[0056] Adopt the method similar to embodiment 1, difference is to increase the consumption of water, promptly replaces 60ml water with 80ml water. 177g of etherified compound and 262g of crude isoflurane were recovered, and the remaining high-fraction substance was reduced by adding isopropanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com