Phosphorus-containing hydroquinone derivative, phosphorus-containing flame-retardant epoxy resin, preparation method and application thereof
A technology of hydroquinone and epoxy resin, which is applied in the field of phosphorus-containing flame retardant epoxy resin and its preparation, can solve the problems such as the influence of the final state of the processing effect on dimensional stability, high heat release, and reduced heat resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
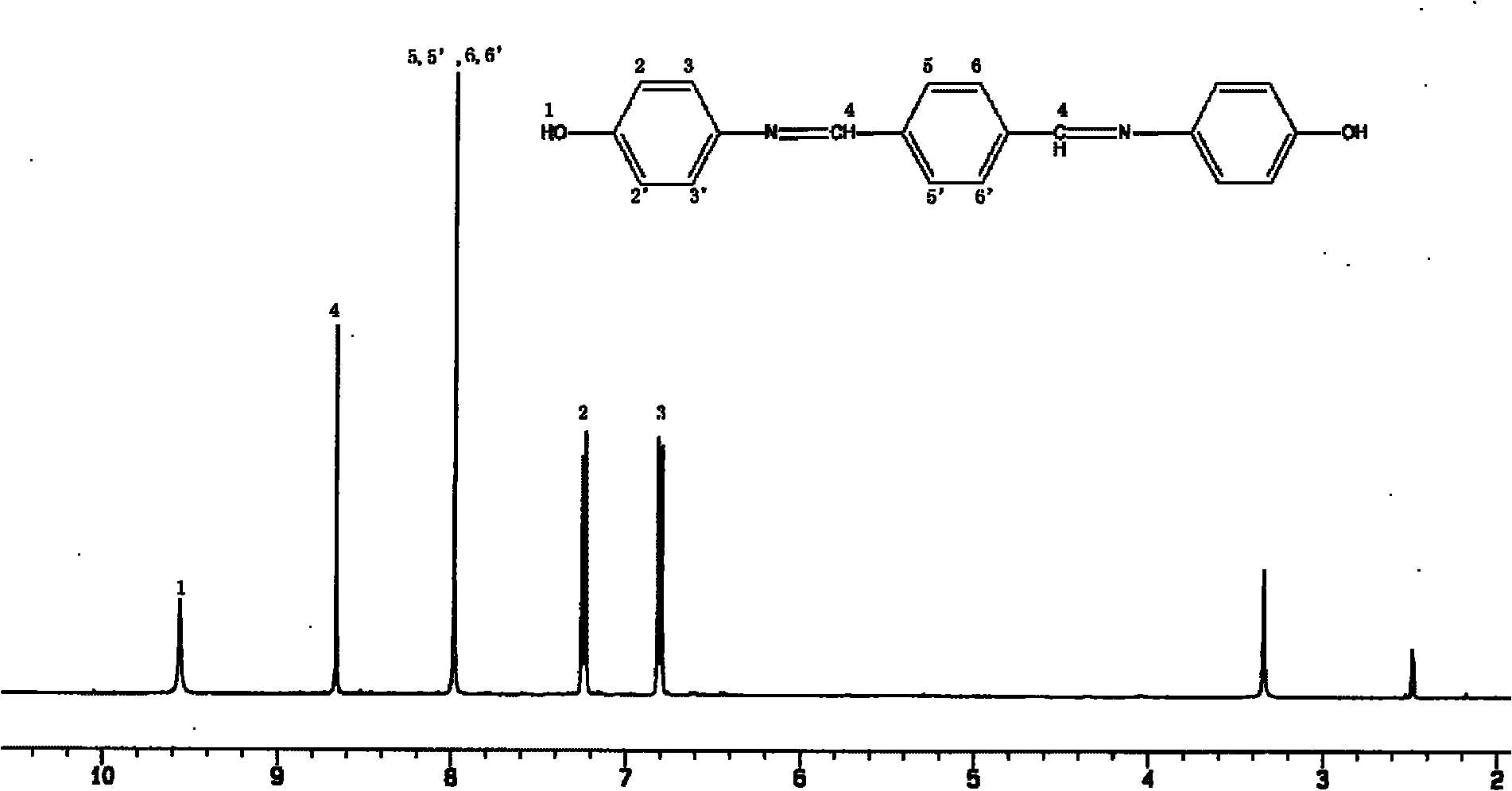
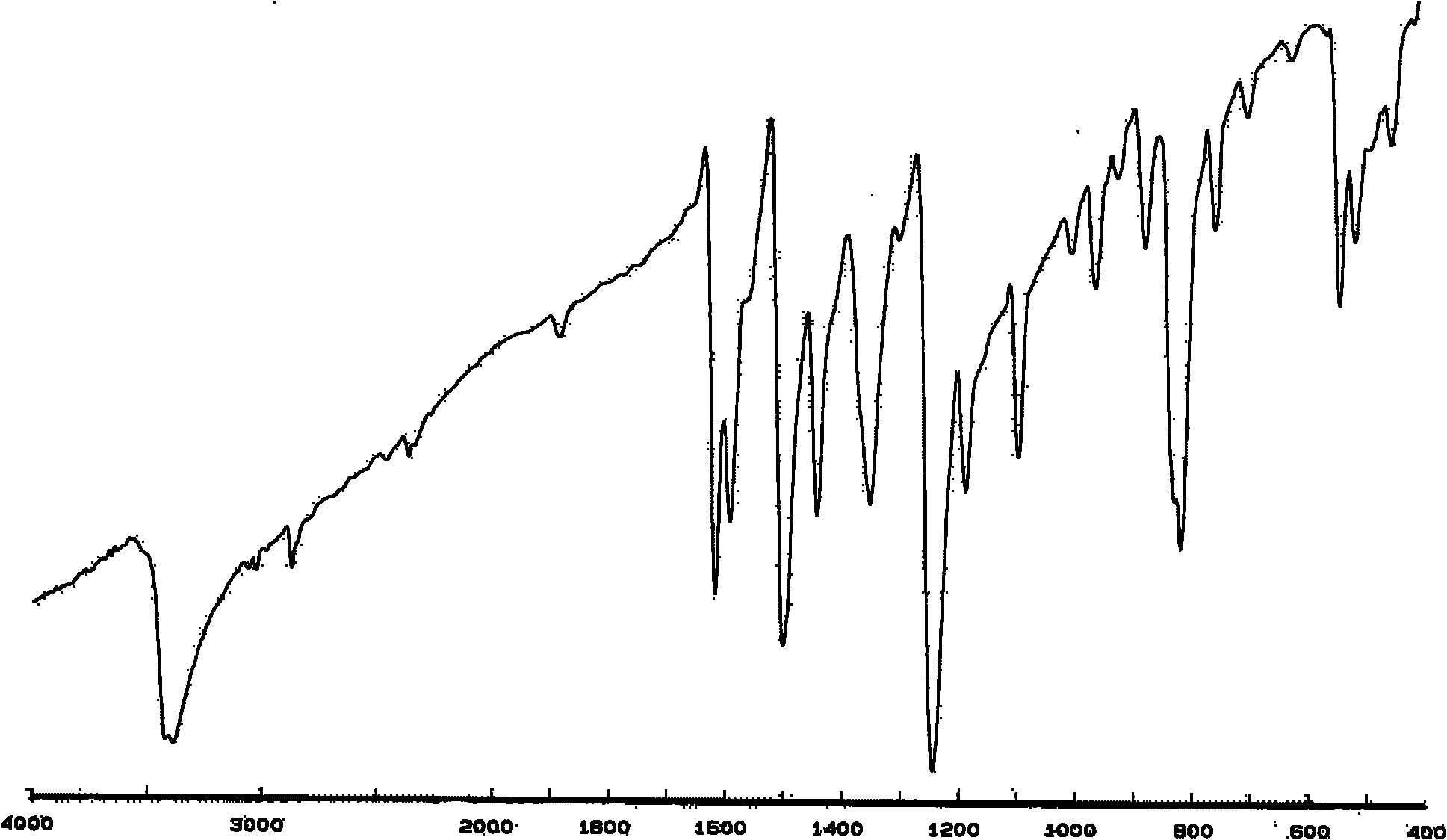
[0077] (1) With N 2 Pass into the reaction flask of device, stirring device, thermometer, condensate pipe, add 20g terephthalaldehyde, 30g p-aminophenol, and dissolve in the mixing of DMSO and ethanol (EtOH) (DMSO:EtOH=1:2 volume ratio) In the solvent, add 0.6g ZnCl 2as a catalyst. The reaction was heated to 80°C, stirred and refluxed for 3 hours, and cooled to room temperature, a large number of yellow crystals precipitated. Suction filtration, washed 3 times with deionized water, placed in a vacuum oven, dried at 75°C for 24 hours, and the product obtained was recrystallized with a mixed solvent of ethanol / DMSO (8:1) to obtain 46g of Phenolic hydroxyl group, imine dihydric phenol substance BP-1 with Schiff base structure on the skeleton. pass 1 H NMR and FT-IR detection methods, the chemical structure of the synthesized compound BP-1 has been relatively clearly characterized, and the results are respectively figure 1 and figure 2 shown.
[0078] (2) Add 31.6g BP-1, 9...
Embodiment 2
[0080] (1) With N 2 Pass into the reaction flask of device, stirring device, thermometer, condensate pipe, add 20g terephthalaldehyde, 30g p-aminophenol, and dissolve in the mixing of DMSO and ethanol (EtOH) (DMSO:EtOH=1:2 volume ratio) In the solvent, add 0.6g ZnCl 2 as a catalyst. The reaction was heated to 80°C, stirred and refluxed for 3 hours, and cooled to room temperature, a large number of yellow crystals precipitated. Repeat the operation of Example 1 step (1) to obtain 46g BP-1.
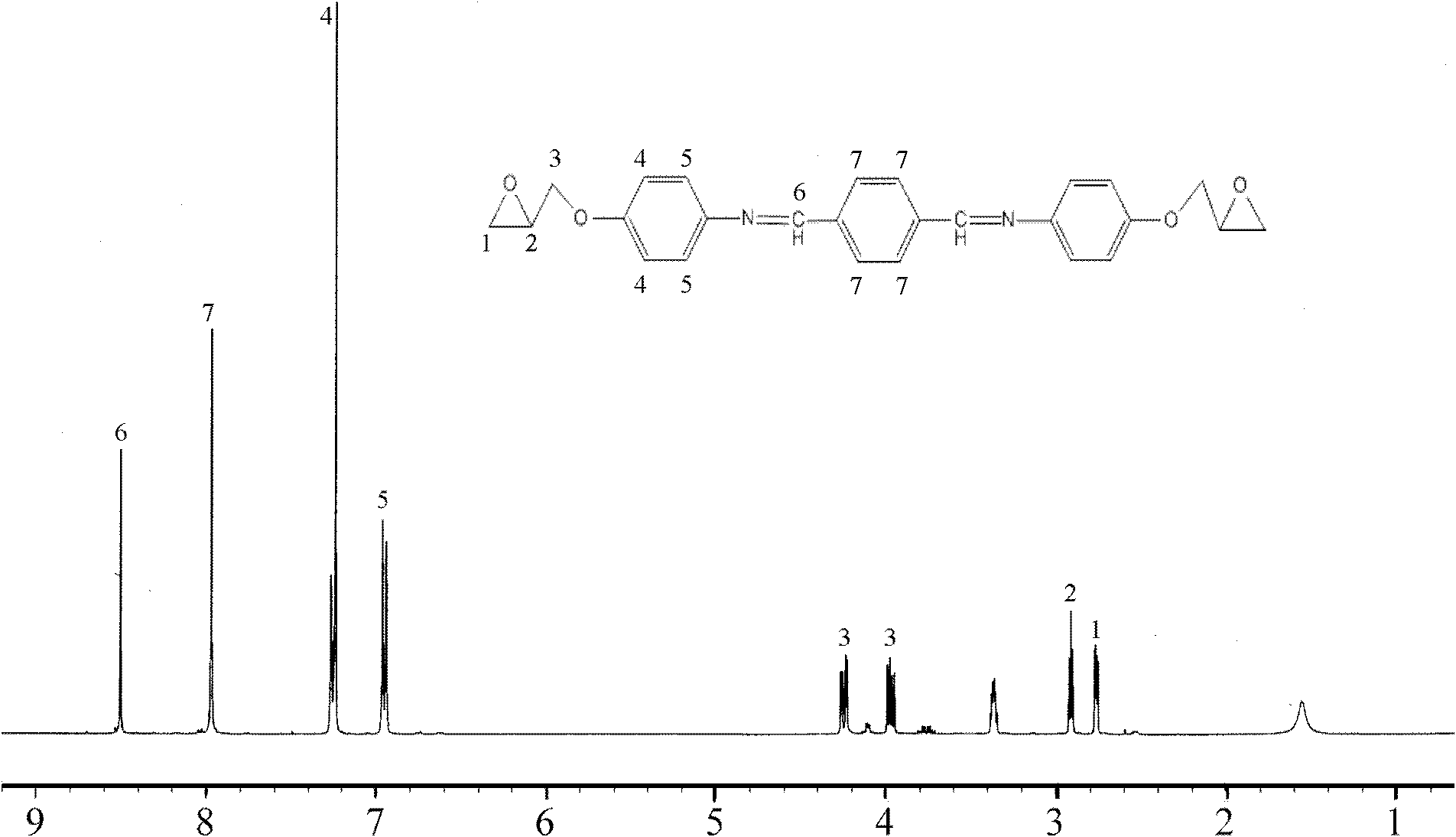
[0081] (2) In another reaction flask equipped with a stirring device, a thermometer, and a condensate pipe, add the BP-145g obtained from the above reaction dissolved in a mixed solvent of ethanol and DMSO (DMSO:EtOH=1:2 volume ratio), heat to 75°C, stir, dissolve, add 0.7g ZnCl 2 as a catalyst. In thru dry N 2 Under the protection of , began to slowly drop 50g diethyl phosphite, and the dropwise addition was completed in 2 hours. Then the temperature was raised to 90°C, and the reac...
Embodiment 3
[0084] According to the epoxy equivalent value of the epoxy resin, weigh them respectively according to the equimolar stoichiometric ratio (1:1): Group A: 296.8 mg of the imine epoxy resin containing the Schiff base structure on the skeleton obtained in Example 1 Monomer EP-1 and 68.8mg curing agent 4,4'-diaminodiphenylmethane (MDA); B group: phosphorus-containing flame-retardant epoxy monomer EP-2 and 31.7mg obtained in 225.2mg embodiment 2 Curing agent 4,4'-diaminodiphenylmethane (MDA); Group C: 832.1mg common epoxy resin EPON-828 (DGEBA) and 218.2mg curing agent 4,4'-diaminodiphenylmethane (MDA ). Then use the mixed solvent of acetone and chloroform with a volume ratio of 1:10 as the solvent, stir and mix the groups A, B and C respectively, and remove the solvent under vacuum at room temperature. The obtained mixture was cured at a high temperature in a vacuum oven, and the curing conditions were: 2 hours at 160°C, 2 hours at 180°C, 2 hours at 200°C, and 1 hour at 220°C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com