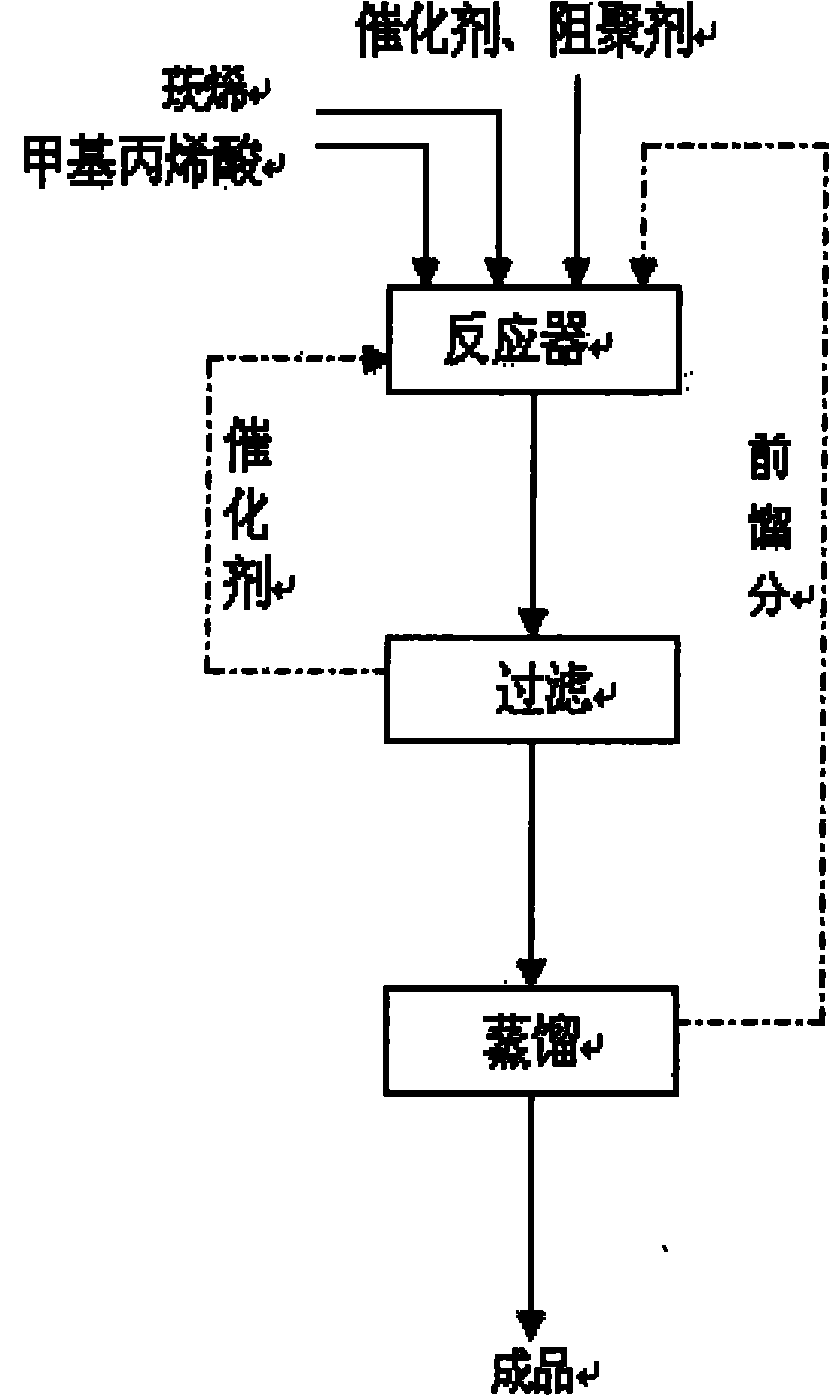
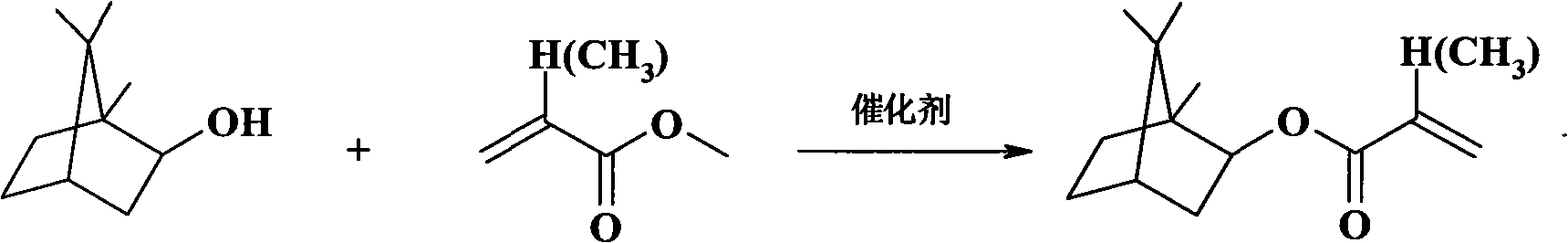
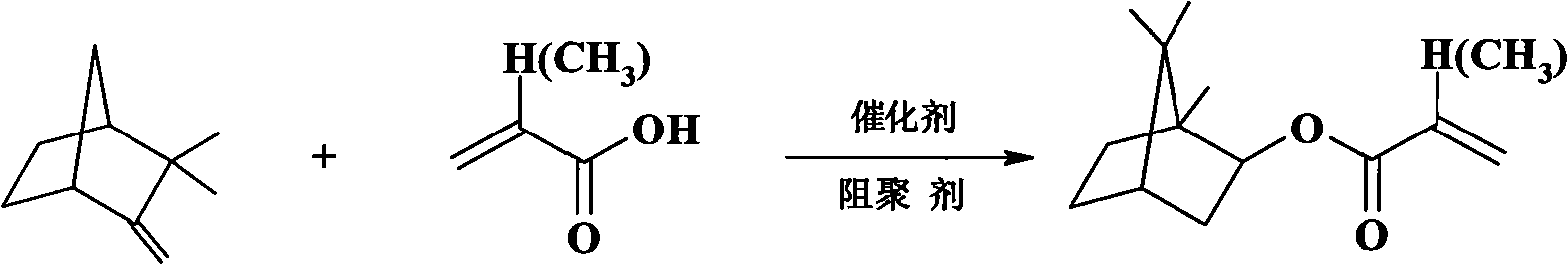
Method for catalytically synthesizing isobornyl methacrylate by activated carbon supported stannic chloride
A technology of isobornyl methacrylate and methacrylic acid is applied in chemical instruments and methods, preparation of organic compounds, catalysts for physical/chemical processes, etc. Overcome the effects of high price, low moisture content and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 135.00g camphene (purity is 74.1%, 0.735mole) and 82.18g methacrylic acid (0.954mole) are pre-mixed and then added to a 500mL four-necked flask equipped with a thermometer, condenser and mechanical stirrer, followed by adding 17.4g Activated carbon supports tin tetrachloride catalyst and 1.2753g of phenothiazine. After the material is kept at 40°C for 1 hour, the temperature is raised to 60°C, and the reaction is carried out at constant temperature in a water bath for 6h, followed by detection by gas chromatography. After the reaction solution was filtered to remove the catalyst, 0.5g Na 2 CO 3 After stirring for 20 minutes, distill under reduced pressure to distill off unreacted camphene and excess methacrylic acid, and collect fractions at 113-120°C / 6mm Hg. The product yield is 77.4%, the reaction selectivity is 94.3%, and the ester content is 99.5%.
Embodiment 2
[0020] 135.00g camphene (purity is 74.1%, 0.735mole) and 88.50g methacrylic acid (1.028mole) (the molar ratio of camphene and methacrylic acid is 1: 1.4) is mixed in advance, dissolves at room temperature, waits to dissolve completely Finally, it was added to a 500mL four-necked flask equipped with a thermometer, a condenser and a mechanical stirrer, and then 22.3g of activated carbon-supported tin tetrachloride catalyst and 0.9425g of phenothiazine were added. After the material was kept at 40°C for 1 hour, the temperature was raised to 50°C, and the reaction was carried out in a constant temperature water bath for 7 hours, followed by detection by gas chromatography. Add 0.6g Na 2 CO 3 And stir for 20min, steam unreacted camphene and excess methacrylic acid under reduced pressure, collect 113~120 ℃ / 6mm Hg fraction, be isobornyl methacrylate, product yield 83.9%, reaction selection The property is 95.3%, and the ester content is 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com