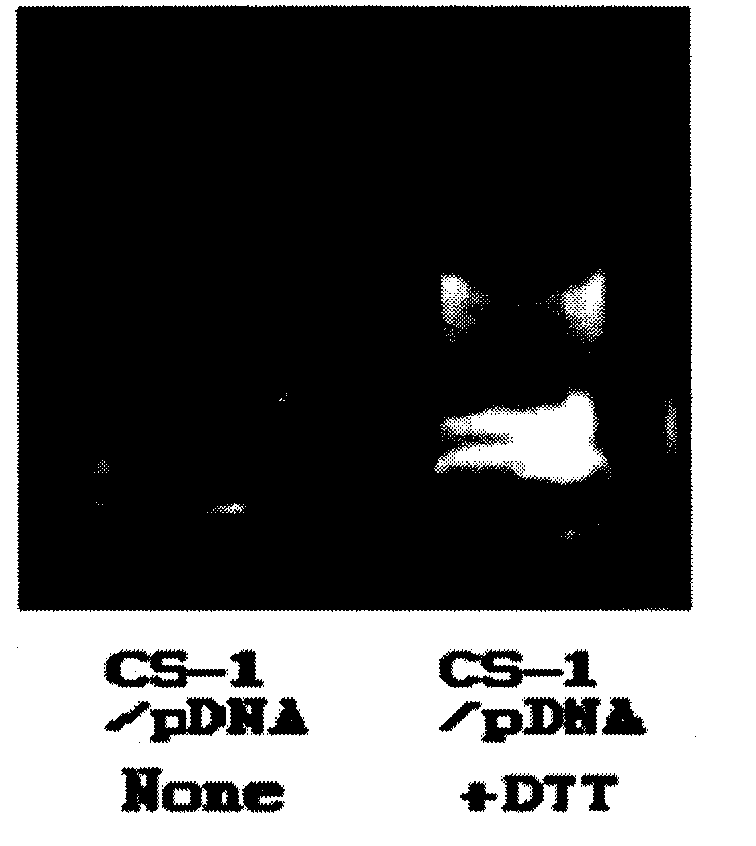
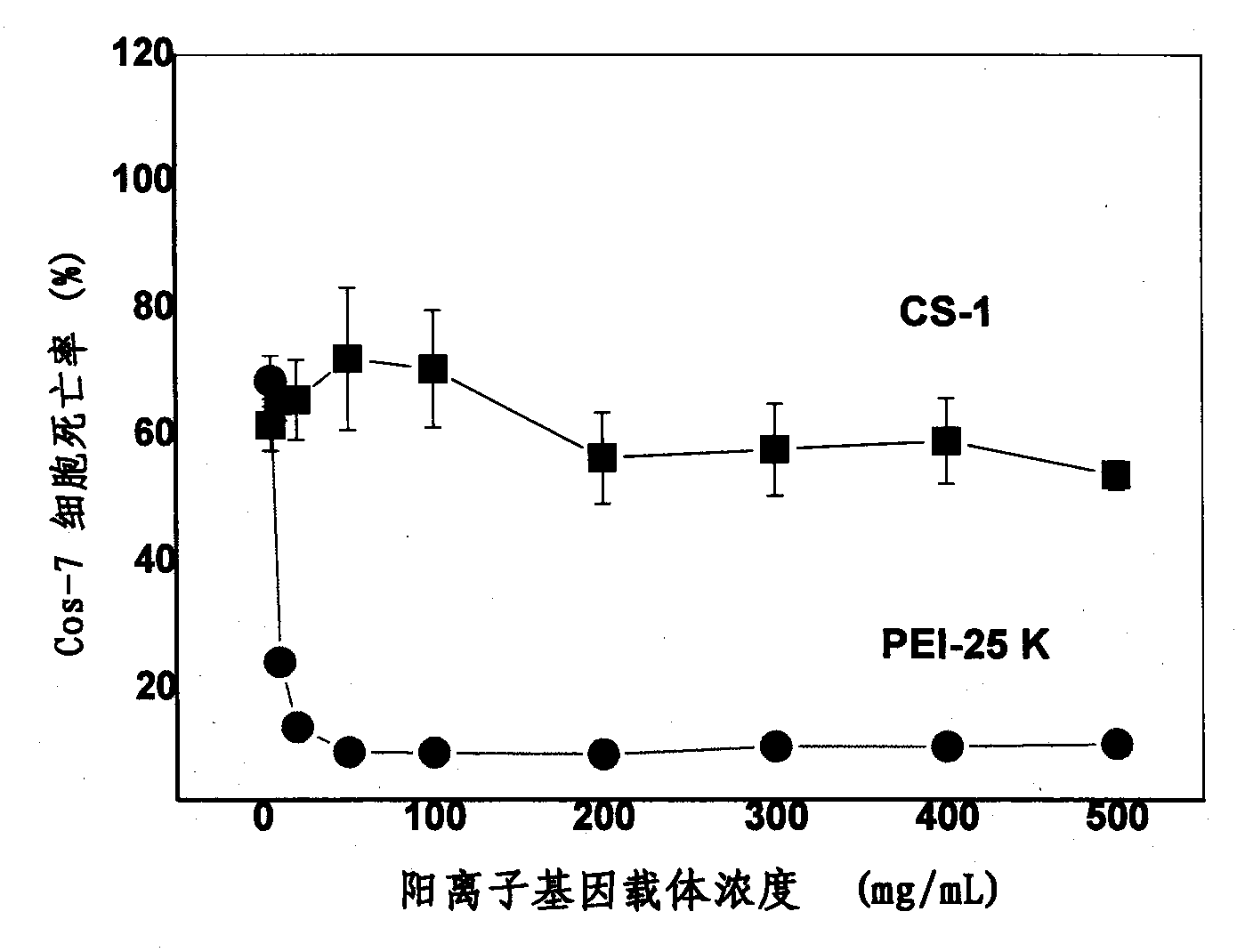
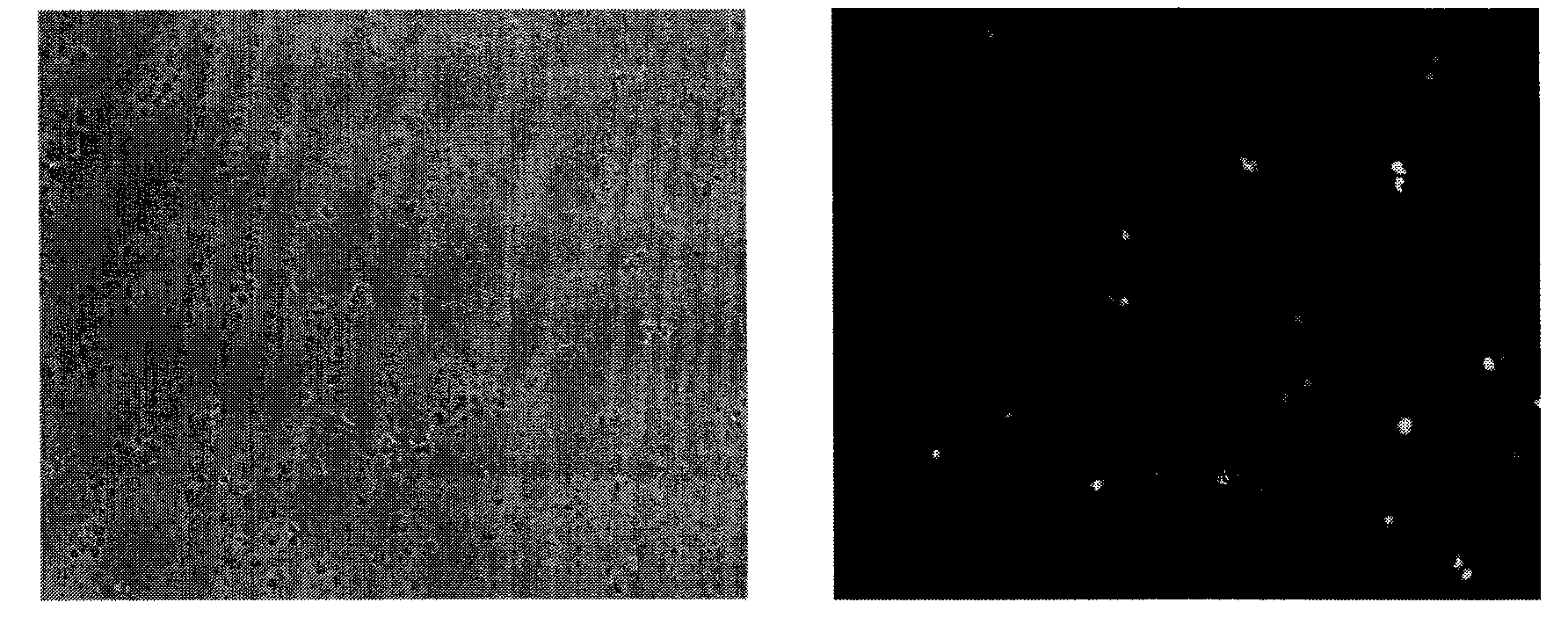
Organic functional compound having disulfide chemical bond and steroids skeleton, preparation method thereof and use thereof
A technology of skeleton structure and disulfide chemistry, which is applied in the application field of functional surfactants, can solve the problems of complicated synthesis and lack of in vivo environment responsiveness of the carrier, and achieves simple synthesis method, good biological reducibility, good purity and water solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] (1) Compound CS-1
[0041] The first step: Dissolve bis(2-hydroxyethyl)-disulfide compound (14g, 90mmol) in 200ml dry tetrahydrofuran, slowly add 4-nitrate dissolved in 50ml dry tetrahydrofuran dropwise under the catalysis of 20ml triethylamine Phenyl chloroformate (12g, 60mmol), reacted in an ice bath at 0°C for 30h, then distilled off tetrahydrofuran under reduced pressure, purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:1 (v / v )), the monohydroxy disulfide compound intermediate is prepared, and its productive rate is 35%, and the main chemical structure features are as follows:
[0042] 1 H NMR (CDCl 3 , 300MHz): 8.27(m, 2H, ArH), 7.37(m, 2H, ArH), 4.56(t, 2H, J=6.3Hz, CH 2 OCO), 3.91(t, 2H, J=6.6Hz, CH 2 OH), 3.03(t, 2H, J=5.7Hz, CH 2 S), 2.89(t, 2H, J=5.7Hz, CH 2 S).
[0043] ESI-MS: [M + ]=319.1m / z
[0044] The second step: the monohydroxy disulfide intermediate (3.2g, 10mmol) prepared above was dissolved in 30ml of ...
Embodiment 2
[0051]
[0052] (2) Compound CS-2
[0053] The first step: Dissolve two (2-hydroxyethyl)-disulfide compounds (28g, 180mmol) in 500ml of dry anhydrous acetonitrile, and slowly drop them under the catalysis of 10g 4-dimethylaminopyridine (DMAP) to dissolve in advance 4-Nitrochloroformic acid phenol ester (25g, 60mmol) in 50ml of dry chloroform, react at room temperature for 15h and remove chloroform under reduced pressure, through column chromatography (eluent: dichloromethane: petroleum Ether=1: 1 (v / v)) purification, obtains the intermediate containing disulfide chemical bond, and its productive rate is 43%, and main chemical structural feature is as follows:
[0054] 1 H NMR (CDCl 3 , 300MHz): 8.27(m, 2H, ArH), 7.37(m, 2H, ArH), 4.56(t, 2H, J=6.3Hz, CH 2 OCO), 3.91(t, 2H, J=6.6Hz, CH 2 OH), 3.03(t, 2H, J=5.7Hz, CH 2 S), 2.89(t, 2H, J=5.7Hz, CH 2 S).
[0055] ESI-MS: [M + ]=319.1m / z
[0056] The second step: the disulfide intermediate (7g, 13mmol) prepared in the p...
Embodiment 3
[0063]
[0064] (3) Compound CS-3
[0065] The first step: Dissolve bis(2-hydroxyethyl)-disulfide compound (28g, 180mmol) in 500ml dry anhydrous acetonitrile, under the catalysis of 10g 4-dimethylaminopyridine (DMAP), slowly dropwise add Dissolve 4-nitrochloroformic acid phenol ester (25g, 60mmol) in 50ml of dry chloroform, react at room temperature for 15h, remove chloroform by distillation under reduced pressure, and go through column chromatography (eluent: dichloromethane: Petroleum ether=1:1 (v / v)) separation and purification, the intermediate that contains disulfide chemical bond is prepared, and its productive rate is 43%, and main chemical structure characteristic is:
[0066] 1 H NMR (CDCl 3 , 300MHz): 8.27(m, 2H, ArH), 7.37(m, 2H, ArH), 4.56(t, 2H, J=6.3Hz, CH 2 OCO), 3.91(t, 2H, J=6.6Hz, CH 2 OH), 3.03(t, 2H, J=5.7Hz, CH 2 S), 2.89(t, 2H, J=5.7Hz, CH 2 S).
[0067] ESI-MS: [M + ]=319.1m / z
[0068] The second step: the disulfide compound intermediate (7g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com