Cobalt nitride compound and preparation method thereof as well as methanol fuel cell catalyst and preparation method thereof
A cobalt nitride compound, methanol fuel cell technology, applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, nitrogen-metal/silicon/boron binary compound, etc., can solve uneven distribution and increase electrochemical reaction speed , large catalyst particle size, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] In the preparation method of the above-mentioned cobalt nitride compound, the reaction raw material cobalt acetate with a particle size of 300-500nm is sintered at high temperature in the presence of a nitrogen source atmosphere to obtain the cobalt nitride compound.
[0018] A methanol fuel cell catalyst, the catalyst contains the above-mentioned cobalt nitride compound and a carrier for supporting the cobalt nitride compound.
[0019] The carrier is known to those skilled in the art, such as acetylene black, activated carbon, superconducting carbon black, preferably Vulcan X-72R carbon powder.
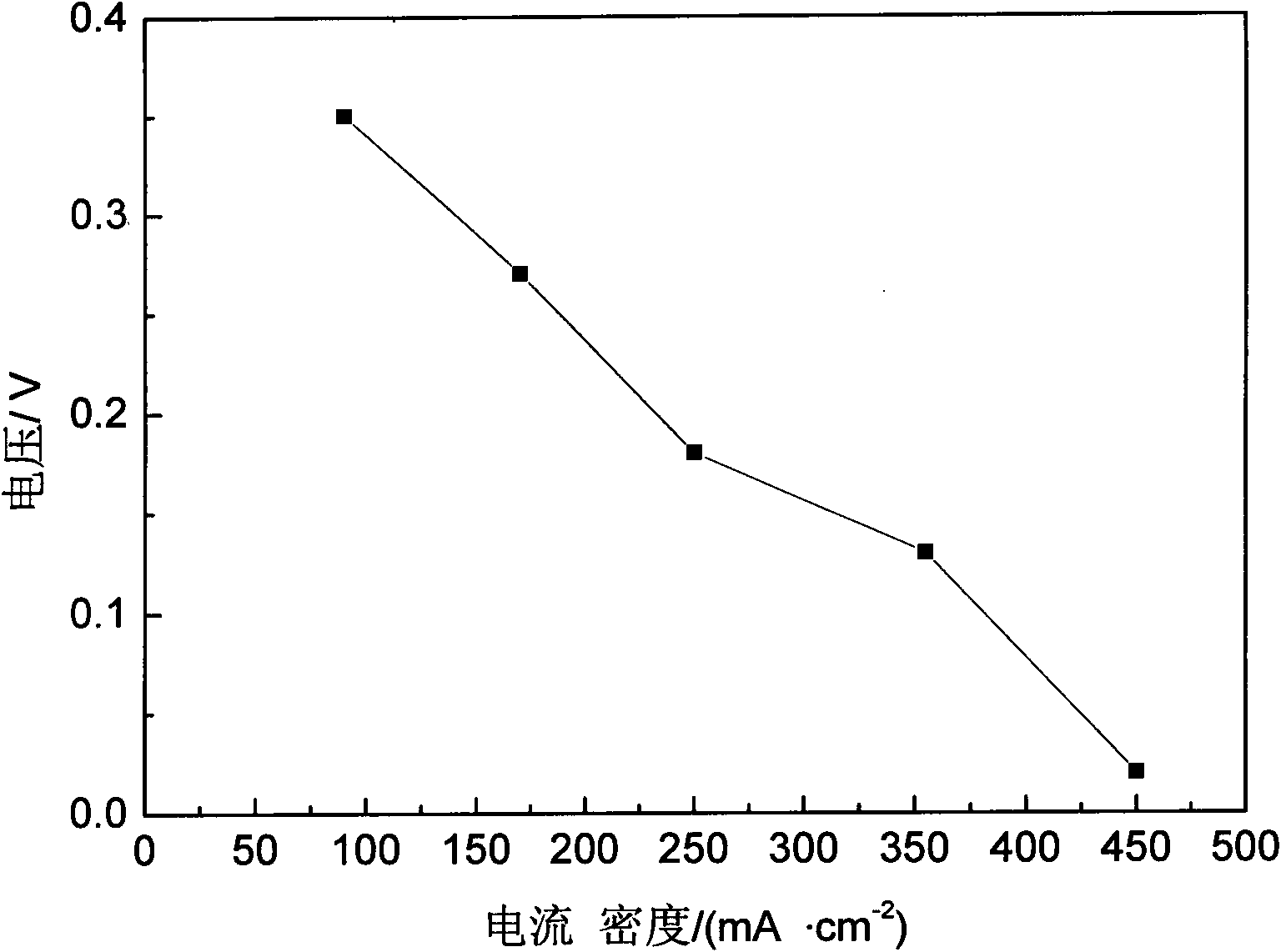
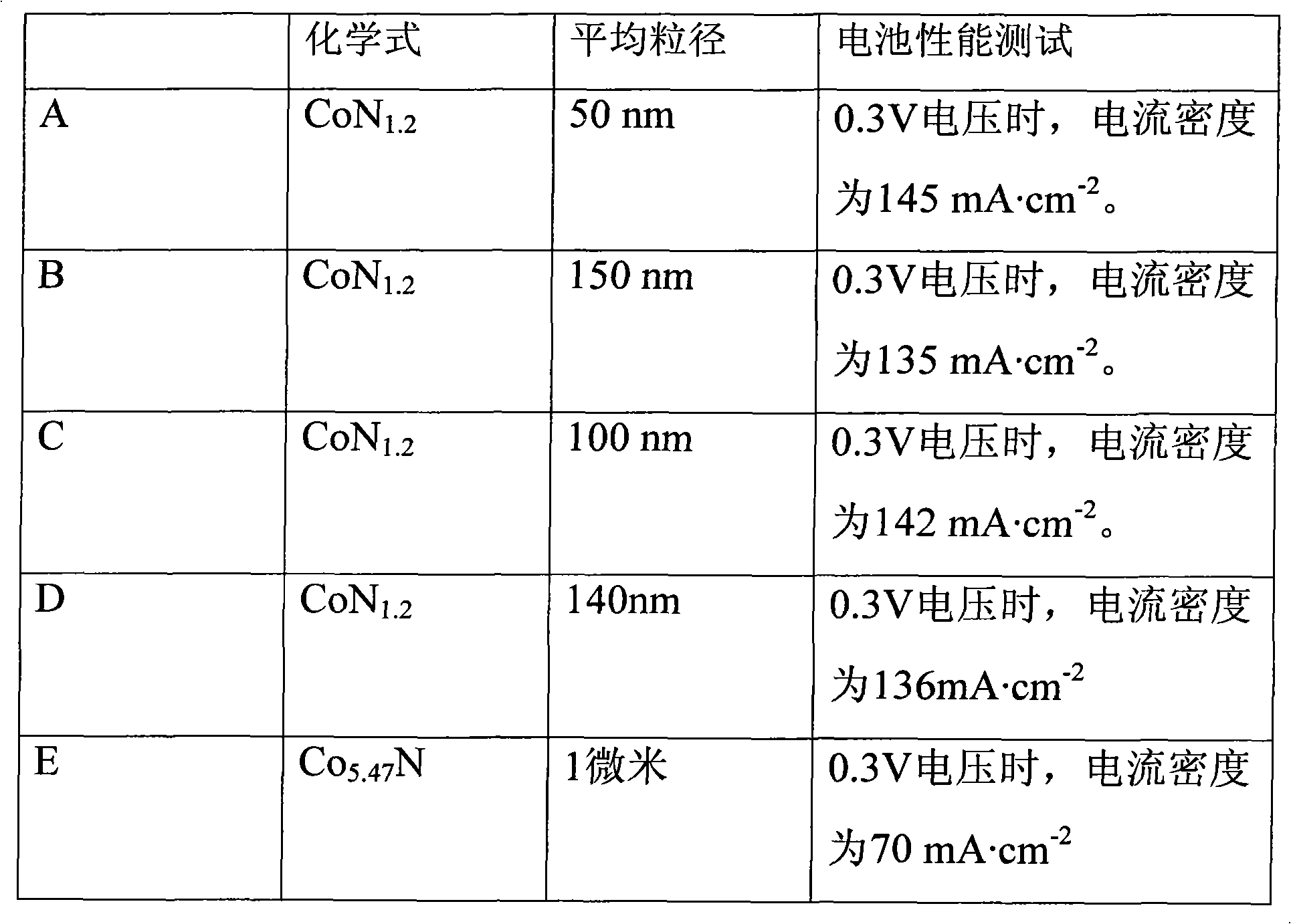
[0020] The prepared catalyst has an average particle diameter of 100nm-200nm.
[0021] The cobalt nitride compound and the carrier used to support the cobalt nitride compound are used as a reference, wherein the mass percentage of the cobalt nitride compound is 30wt%-40wt%.
[0022] The equation of reaction of the present invention is:
[0023] Co(CH 3 COO) 2 →Co(C 2 o 4...
Embodiment 1
[0042] Average particle size is 400nm cobalt acetate Co (Ac) 4.48g is put into the porcelain boat, and the porcelain boat is put into the tube furnace, after getting rid of the air in the tube furnace with NH3, continue to pass into NH3, furnace temperature Raise to 450°C and keep the temperature for 1 hour, then raise the furnace temperature to 800°C and keep the temperature for 2 hours. After the furnace temperature drops to room temperature, stop feeding NH3 to obtain cobalt nitride compound A.
Embodiment 2
[0044] 1. Pretreatment of carrier carbon black
[0045] Weigh 5g of carbon black and soak it in concentrated hydrochloric acid solution to remove possible chloride impurities. After washing, put it into concentrated nitric acid solution to soak and oxidize. Wash with a large amount of deionized water and then dry it.
[0046] 2. Preparation of catalyst
[0047] Weigh 4.48 g of cobalt acetate Co(Ac) and 3.12 g of pretreated carbon black, put them into a beaker filled with 100 mL of distilled water, stir for 2 hours, dry the water in an oven, and perform high-speed ball milling in an anhydrous ethanol medium 2h, put it into a tube furnace until the average particle size is 400nm, and sinter at high temperature with the method of Example 1 to obtain catalyst B.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com