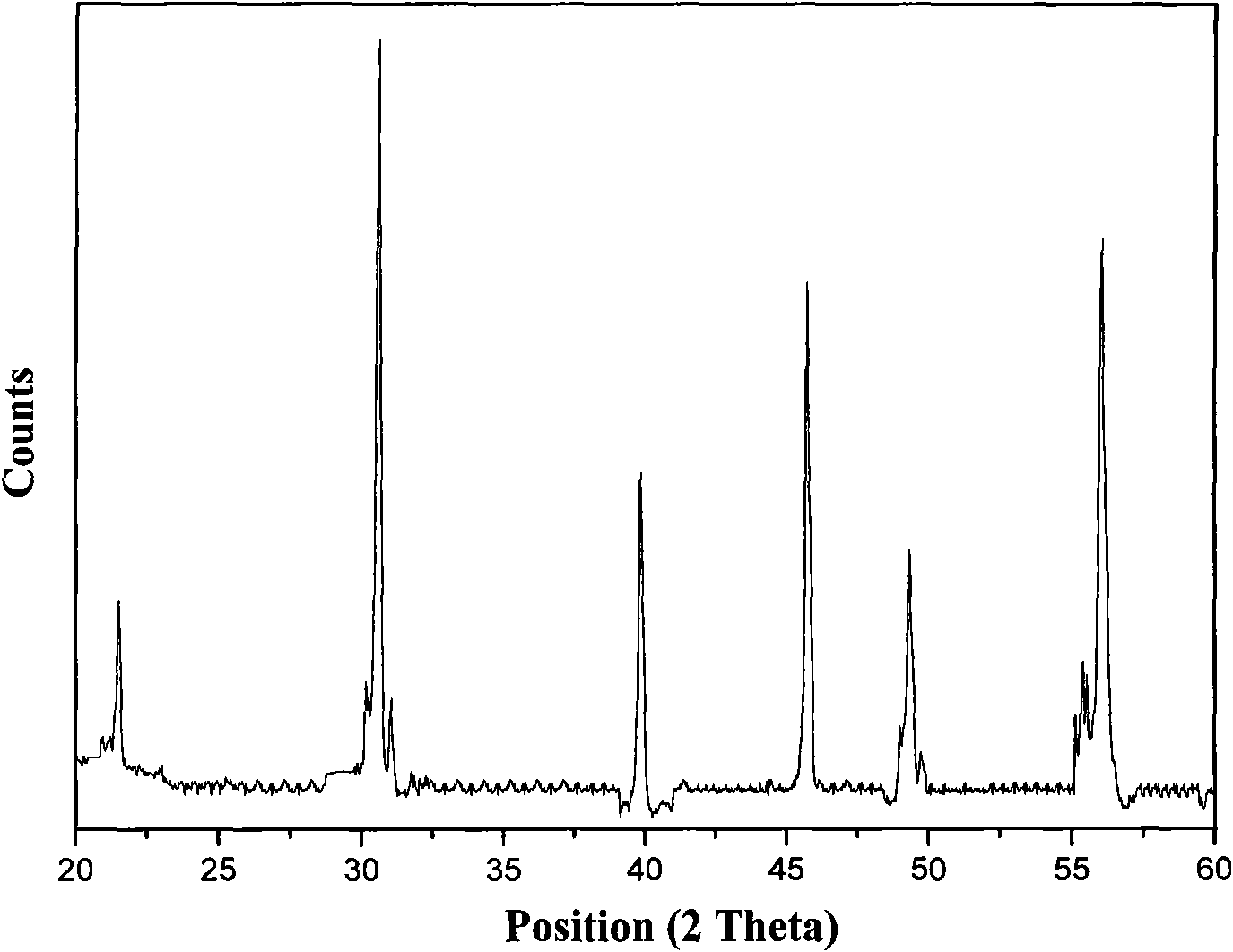
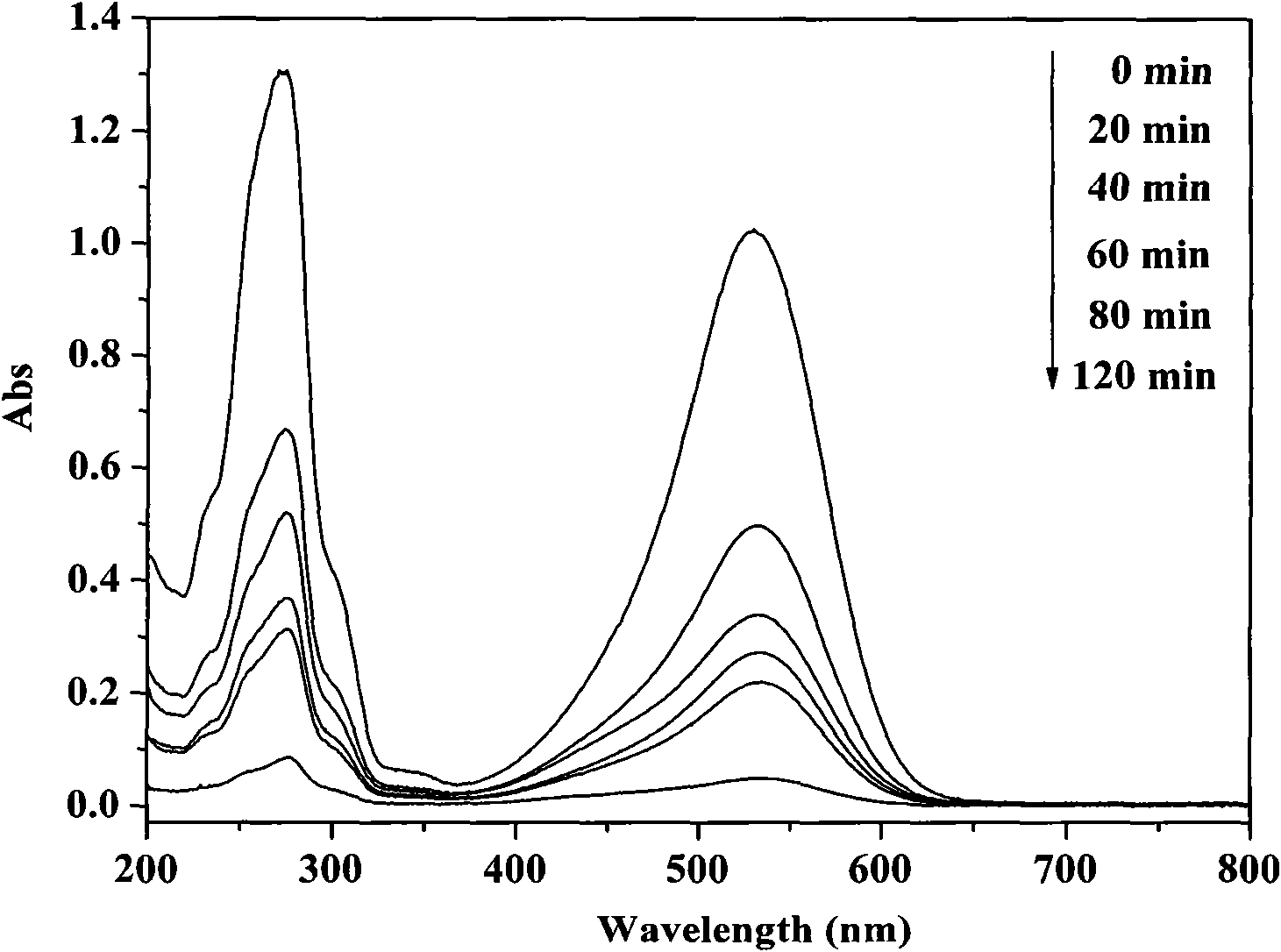
Method for preparing bismuth ferrite photocatalyst
A technology of photocatalyst and bismuth ferrite, which is applied in the field of environmental science and engineering, can solve the problems of unsolvable catalyst separation, small particle size of bismuth ferrite material, and unresearched photocatalytic activity, so as to facilitate industrialization promotion and process cycle Short, not easy to secondary pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take by weighing 9.2 grams of P123 block copolymer surfactants, and dissolve it in 100 milliliters of acetic acid (CH 3 COOH) solution to form a concentration of 0.016 mol / liter solution, then weighed 34 grams of bismuth nitrate pentahydrate (Bi(NO 3 ) 3 ·5H 2 O) join in the above-mentioned solution, after dissolving completely, add 2.36 grams of ferric nitrate (Fe(NO 3 )3 9H 2 (2), the molar ratio of bismuth nitrate and ferric nitrate was 12:1 at this moment; By magnetic stirring, the raw material was completely dissolved and became a uniform sol; under stirring, the sol was aged at room temperature for 3 hours to obtain brown Precursor solution; the precursor solution is transferred to a stainless steel reaction kettle with polytetrafluoroethylene lining, the filling degree is 80%, the reaction kettle is sealed, and the reaction kettle is placed in a 150°C electric constant temperature blast drying oven for crystallization Allow it to fully react for 24 hours; t...
Embodiment 2
[0029] Take by weighing 9.2 grams of P123 block copolymer surfactant, it is dissolved in 100 milliliters of acetic acid (CH 3 COOH) solution to form a concentration of 0.016 mol / liter solution, then weighed 34 grams of bismuth nitrate pentahydrate (Bi(NO 3 ) 3 ·5H 2 O) join in the above-mentioned solution, after dissolving completely, add 2.36 grams of ferric nitrate (Fe(NO 3 ) 3 9H 2 (2), the molar ratio of bismuth nitrate and ferric nitrate was 12:1 at this moment; By magnetic stirring, the raw material was completely dissolved and became a uniform sol; under stirring, the sol was aged at room temperature for 3 hours to obtain brown Precursor solution; the precursor solution is transferred to a stainless steel reaction kettle with polytetrafluoroethylene lining, the filling degree is 80%, the reaction kettle is sealed, and the reaction kettle is placed in a 120°C electric constant temperature blast drying oven for crystallization Allow it to fully react for 24 hours; ...
Embodiment 3
[0031] Take by weighing 4.6 grams of P123 block copolymer surfactant, under stirring state, it is dissolved in 100 milliliters of acetic acid (CH 3 COOH) solution to form a concentration of 0.008 mol / liter solution, then weighed 34 grams of bismuth nitrate pentahydrate (Bi(NO 3 ) 3 ·5H 2 O) join in the above-mentioned solution, after dissolving completely, add 2.36 grams of ferric nitrate (Fe(NO 3 ) 3 9H 2 (2), by magnetic stirring, the raw material is completely dissolved and becomes a uniform sol; under stirring, the sol is aged at room temperature for 3 hours to obtain a brown precursor solution; the precursor solution is transferred to a polytetrafluoroethylene In the lined stainless steel reaction kettle, the filling degree is 80%, seal the reaction kettle, and place the reaction kettle in a 130°C electric constant temperature blast drying oven for crystallization for 24 hours to make it fully react; take out the reaction kettle from the oven, Put it into cooling w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com