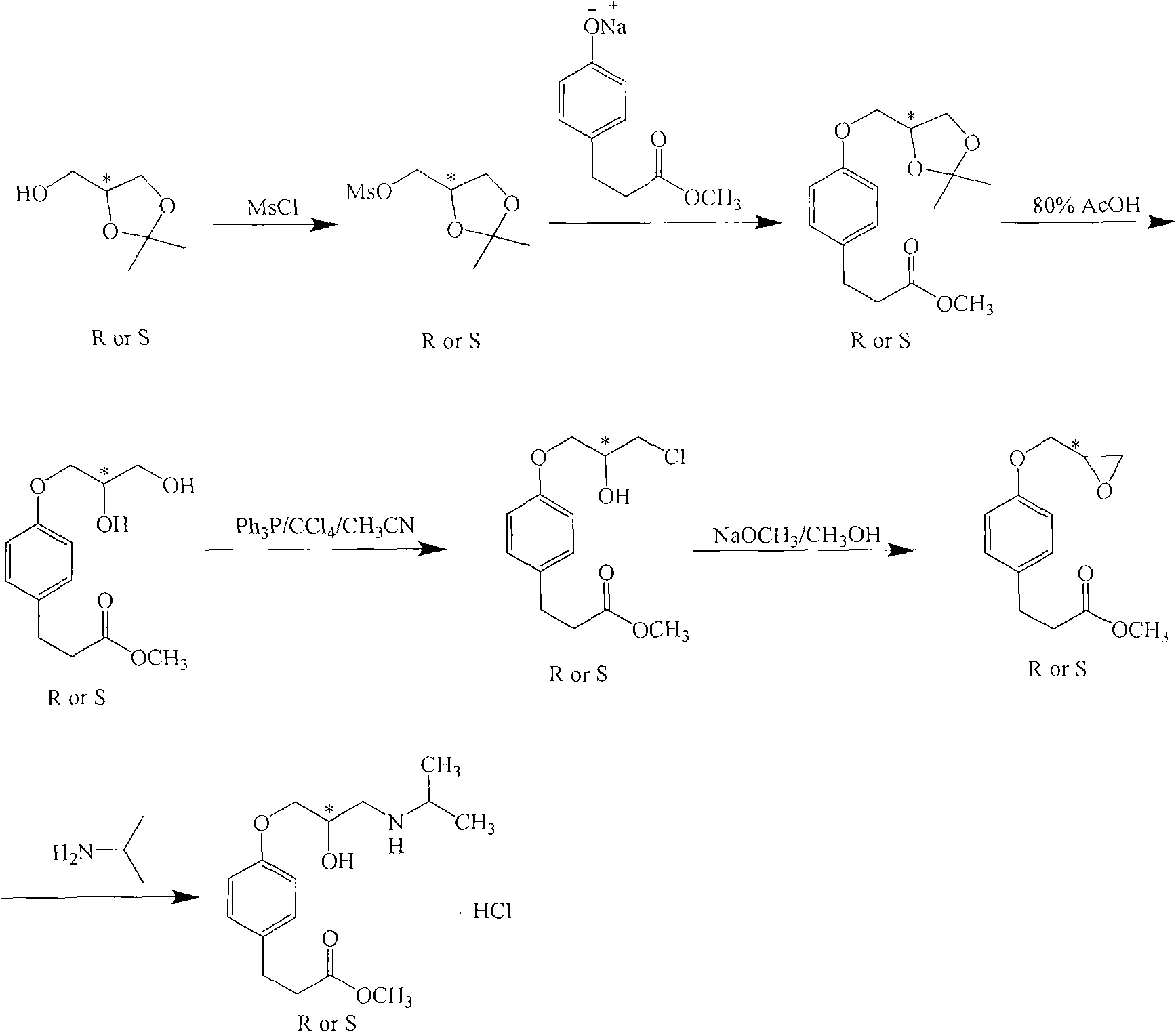
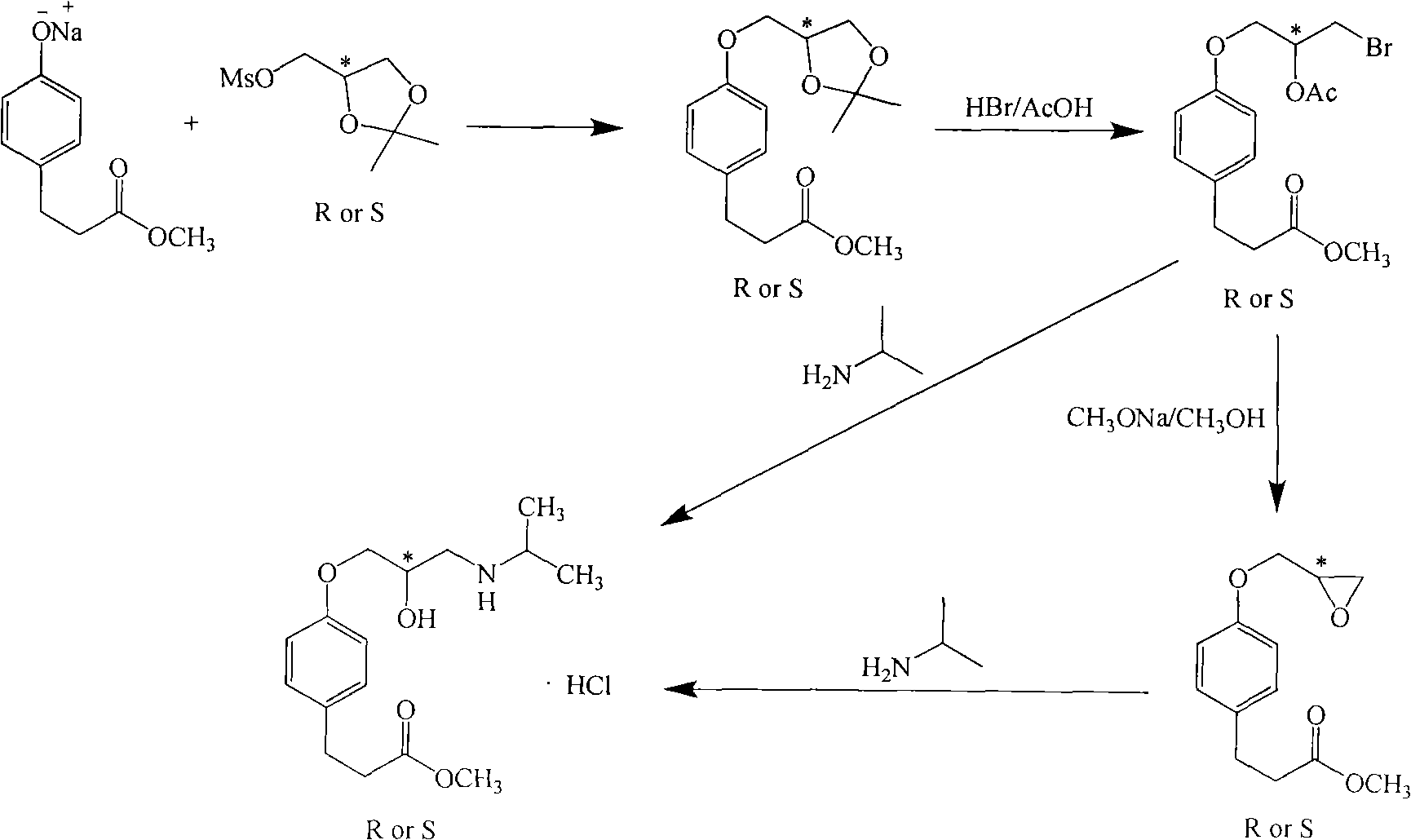
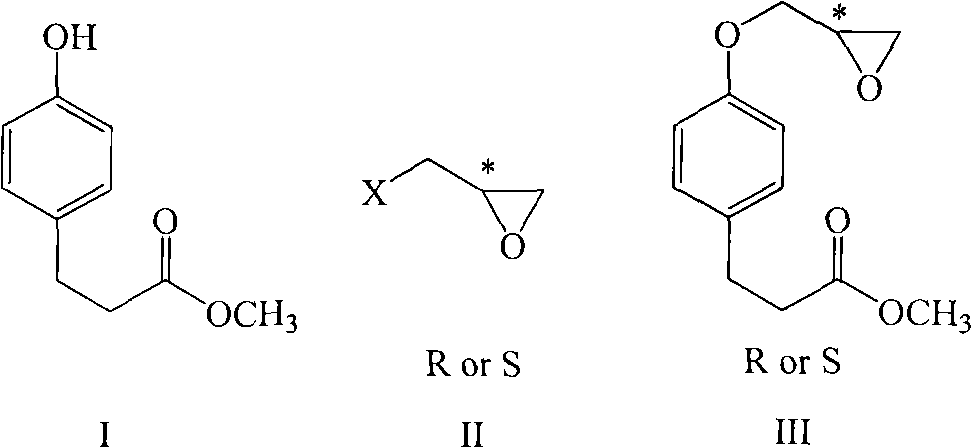
Novel method for preparing esmolol hydrochloride optical isomer
A technology for esmolol hydrochloride and optical isomers, which is applied in the field of preparation of esmolol hydrochloride optical isomers, can solve the problems of high cost, low yield, unsuitability for industrialized large-scale production and the like, and achieves mild conditions, Easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Add 50kg of DMF and 8kg of methyl p-hydroxyphenylpropionate into a dry 100L enamel reaction kettle. After stirring and dissolving, add 0.22kg of 60% NaH in batches at room temperature. Toluenesulfonic acid (S)-glycidyl ester 10.1kg, after adding, continue to react at room temperature, after TLC (ethyl acetate: sherwood oil=1: 10) detection reaction finishes, the reaction solution is poured into the container prepared in advance. 250kg of ice water in a 500L enamel reactor, fully stirred, and then extracted with dichloromethane (100kg×2). The organic layer was dried over anhydrous sodium sulfate, then placed in a 300L enamel reaction kettle and concentrated to dryness under reduced pressure to obtain (S)-methyl 4-glycidoxy phenylpropionate. Add 80kg of methanol and 5.2kg of isopropylamine to the reaction kettle, and react at room temperature. TLC (ethyl acetate:petroleum ether=1:10) showed that after the completion of the reaction, the solvent was recovered under reduced...
Embodiment 2
[0042] Add 50kg of DMF and 8kg of methyl p-hydroxyphenylpropionate into a dry 100L enamel reaction kettle. After stirring and dissolving, add 0.22kg of 60% NaH in batches at room temperature. Toluenesulfonic acid (R)-glycidyl ester 10.1kg, after adding, continue to react at room temperature, after TLC (ethyl acetate: sherwood oil=1: 10) detection reaction finishes, the reaction solution is poured into the container prepared in advance. 250kg of ice water in a 500L enamel reactor, fully stirred, and then extracted with dichloromethane (100kg×2). The organic layer was dried over anhydrous sodium sulfate, then placed in a 300L enamel reaction kettle and concentrated to dryness under reduced pressure to obtain (S)-methyl 4-glycidoxy phenylpropionate. Add 80kg of methanol and 5.2kg of isopropylamine to the reaction kettle, and react at room temperature. TLC (ethyl acetate:petroleum ether=1:10) showed that after the completion of the reaction, the solvent was recovered under reduce...
Embodiment 3
[0045] Add 300g of DMF and 80g of methyl p-hydroxyphenylpropionate into a dry 1L three-necked reaction flask. After stirring and dissolving, add 21.3g of 60% NaH in batches at room temperature. Nitrobenzenesulfonic acid (S)-glycidyl ester 126g, after adding, continue to react at room temperature, TLC (ethyl acetate: sherwood oil=1: 10) detects after reaction finishes, and reaction solution is poured into 1.5kg ice water, Stir well, then extract with dichloromethane (300g×2). The organic layer was dried over anhydrous sodium sulfate, and then concentrated to dryness to obtain (S)-methyl 4-glycidoxyphenylpropionate. 500 g of methanol and 52 g of isopropylamine were added to the raffinate, and the mixture was reacted at room temperature. TLC (ethyl acetate:petroleum ether=1:10) showed that after the reaction was completed, the solvent was recovered under reduced pressure to dryness, then 100 g of methyl acetate was added to the raffinate, the temperature was lowered to below 10°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com