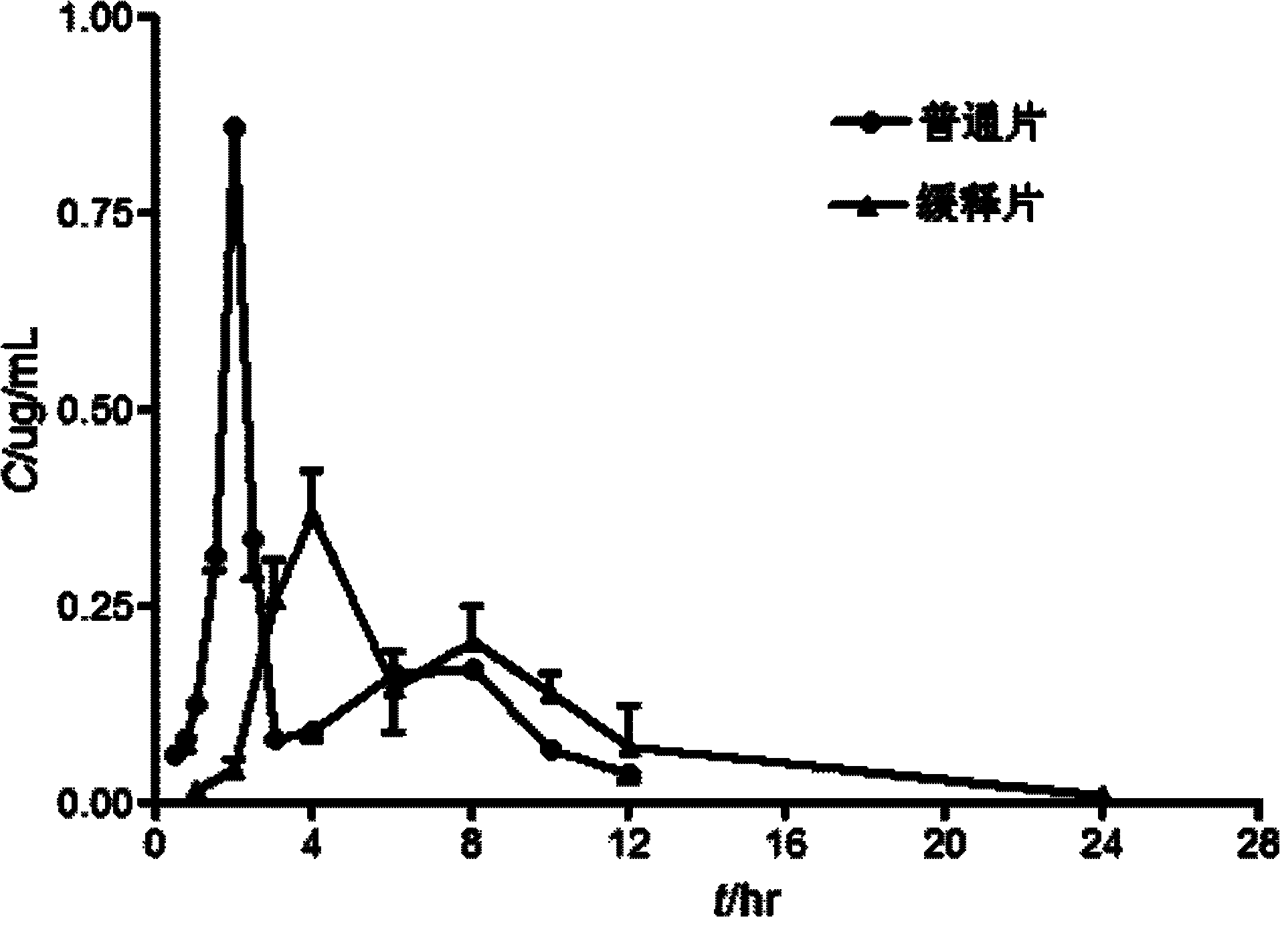
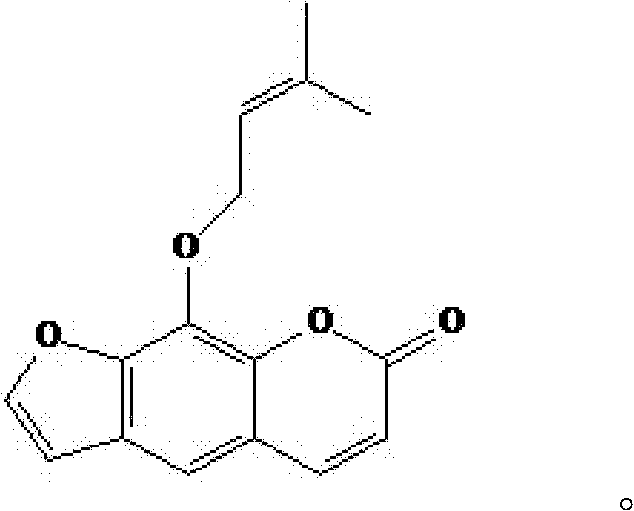
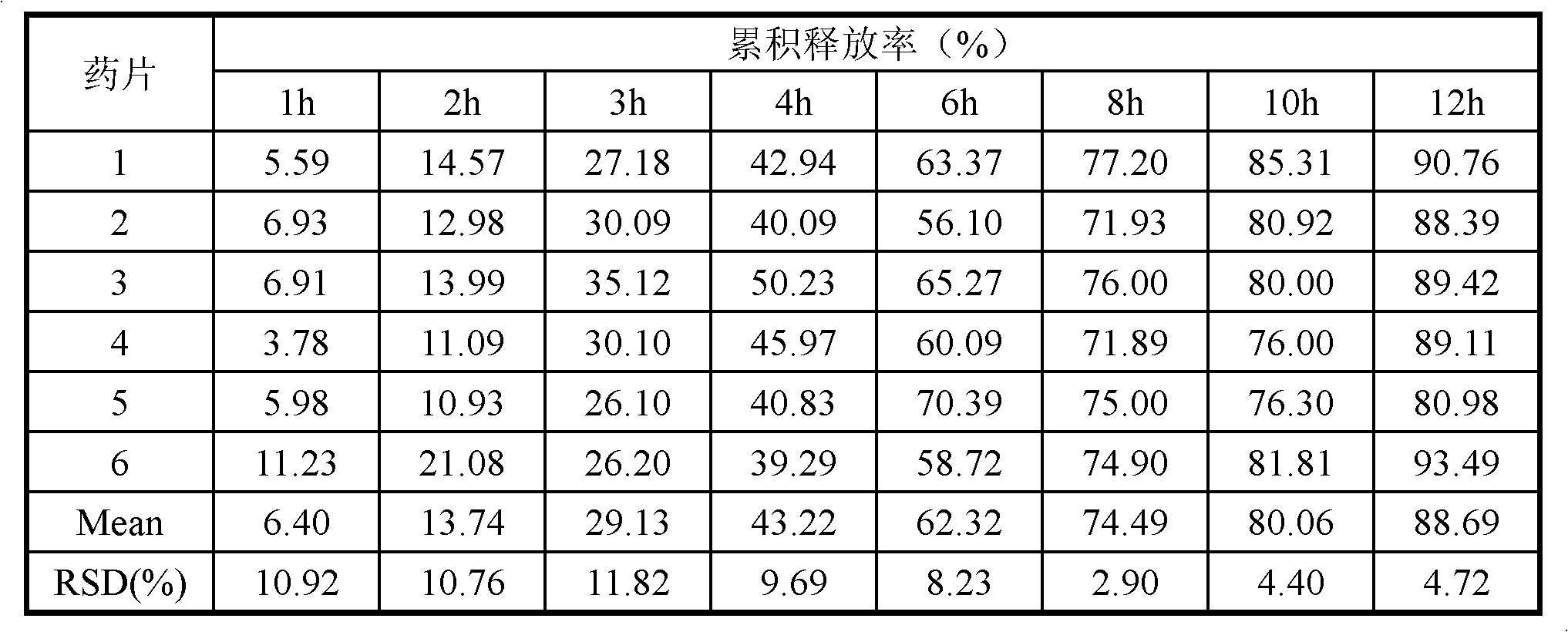
Imperatorin sustained release tablets and preparation method thereof
An imperatorin, sustained-release technology, applied in the field of biomedicine, to achieve the effect of slowing down drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of imperatorin sustained-release tablet comprises the following steps:
[0028] 1) Pass the imperatorin raw material drug and each auxiliary material through an 80-mesh sieve, weigh 50g of imperatorin, 15g of HPMC-100M and 206g of lactose and mix them well, then mix them according to the imperatorin / viscosity Mixture = 10g / 3mL ratio, add 15% (wt) PVP-ethanol-water solution (75%) 15mL, mix thoroughly, pass through a 24 mesh sieve, and wet granulate to obtain wet granules;
[0029] 2) drying the wet granules at 60°C until the water content is 3.0% to obtain dry granules;
[0030] 3) After passing the dry granules through a 24-mesh sieve for granulation, fully mix the dry granules with 24g of carbomer 934NF and 5g of magnesium stearate, and then press them into 1000 tablets to make imperatorin sustained-release tablets .
[0031] Among the made imperatorin sustained-release tablets, in terms of mass fraction, imperatorin is 16.6%, HPMC-100M is 5%, ...
Embodiment 2
[0033] The preparation method of imperatorin sustained-release tablet comprises the following steps:
[0034] 1) Pass the imperatorin crude drug and each auxiliary material through a 80-mesh sieve, weigh 50g of imperatorin, 24g of HPMC-15M and 206g of starch and mix them well, then mix them according to the imperatorin / viscosity Mixture = 10g / 2mL ratio, add 10% (wt) PVP-ethanol-water solution (75%) 10mL, mix thoroughly, pass through a 24 mesh sieve, and wet granulate to obtain wet granules;
[0035] 2) drying the wet granules at 60°C until the water content is 3.0% to obtain dry granules;
[0036] 3) After passing the dry granules through a 24-mesh sieve for grading, fully mix the dry granules with 15g of Carbomer 934NF and 5g of talcum powder, and then press them into 1000 tablets to make imperatorin sustained-release tablets.
[0037] Among the prepared imperatorin sustained-release tablets, in terms of mass fraction, imperatorin is 16.6%, HPMC-15M is 8%, carbomer is 5%, st...
Embodiment 3
[0039] The preparation method of imperatorin sustained-release tablet comprises the following steps:
[0040] 1) Pass the imperatorin raw material drug and each auxiliary material through a 80-mesh sieve, weigh 50g of imperatorin, 30g of HPMC-4M and 200g of dextrin and mix them well, then follow the imperatorin / Adhesive = 10g / 2mL ratio, add 10mL of 60% ethanol, mix thoroughly, pass through a 24-mesh sieve, and wet granulate to obtain wet granules;
[0041] 2) drying the wet granules at 50°C until the water content is 2.5% to obtain dry granules;
[0042] 3) After passing the dry granules through a 24-mesh sieve for grading, fully mix the dry granules with 15 g of polyvinylpyrrolidone and 5 g of talcum powder, and then press them into 1000 tablets to make imperatorin sustained-release tablets.
[0043]Among the prepared imperatorin sustained-release tablets, in terms of mass fraction, imperatorin is 16.7%, HPMC-4M is 10%, polyvinylpyrrolidine is 5%, dextrin is 66.6%, and talc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com