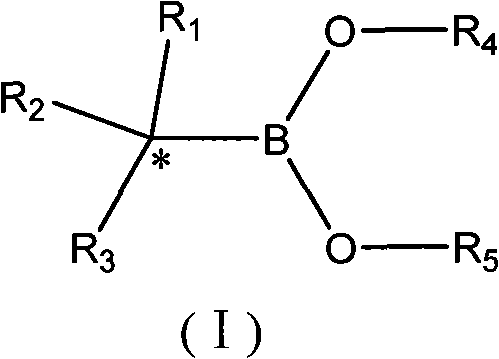
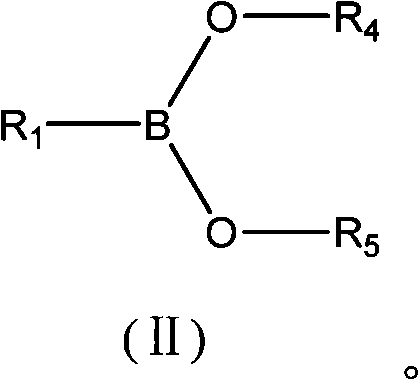
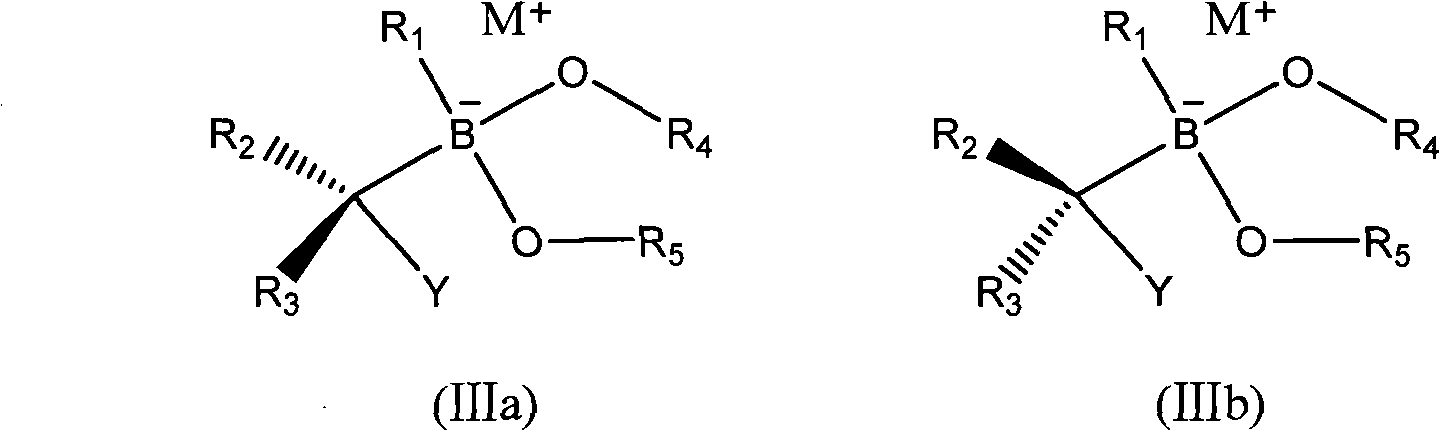
Synthesis technology of alpha-chiral boric acid and boric acid ester
A boric acid ester and chiral technology, applied in the field of α-chiral boronic acid compounds, can solve the problems of increasing economic pressure on the country and patients, lack of effective treatment for patients, and increase in production costs, achieving low cost and total production. The effect of high rate and high overall yield of the route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Example 1: Synthesis of α-chiral borate with lower cost, easier control of process conditions and higher product purity: (1S)-(S)-pinanediol 1-chloro-3-methylbutane Base-1-boronate scale-up process:
[0206]
[0207]Step 1. Add 428 g of isopropyl boric acid and 1.5 kg of methyl tert-butyl ether into a 10-liter reaction vessel, and add a solution comprising 644 g of (S)-pinanediol and 1.5 kg of methyl tert-butyl ether under stirring. After the dropwise addition, continue to stir for more than 2 hours to track the reaction by TLC or GC, and confirm that all the (S)-pinanediols have reacted completely. Add 200 g of saturated aqueous sodium bicarbonate solution to the reaction solution for washing, and separate the liquids; add 200 g of water to wash the organic phase, and separate the liquids; add 200 g of saturated aqueous sodium chloride solution to wash the organic phase. Add 200g of anhydrous sodium sulfate and stir for more than 2 hours, filter to obtain (S)-pinan...
Embodiment 2
[0214] Embodiment two: lower cost, easier control of the conditions described in the process, and higher product purity Synthesis of α-aminoboronic acid ester or its salt with acid: (1R)-(S)-pinanediol Ammonium 1-trifluoroacetate-3-methylbutanyl-1-boronate scale-up process:
[0215]
[0216] Step 4. Synthesize (1S)-(S)-pinanediol 1-chloro-3-methylbutanyl-1-boronate 980g according to steps 1 to 3 in Example 1 and dissolve it into 3.0kg methyl ring In hexane; control the reaction temperature at about minus 20°C and add dropwise to a solution including 338g LiHMDS, 3.3kg of tetrahydrofuran and 1.0kg of n-hexane, and finish adding in about 1.0 hour. After the addition, control the temperature at about minus 10°C and continue stirring for 1.0 hour . After warming up to room temperature, 170g of diatomaceous earth and 2.0kg of methylcyclohexane were added. Rotary evaporation under reduced pressure until the tetrahydrofuran in the system was added. After adding 3.0 kg of methylc...
Embodiment 3
[0220] Embodiment 3: lower cost, easier control of process conditions, higher product purity Synthetic free boronic acid compound comprises dipeptide borate protease inhibitor bortezomib (Bortezomib)
[0221]
[0222] Or its boric acid anhydride (XXVII) can be applied to the improved process of mass production:
[0223]
[0224] Step 6. Synthesize (1R)-(S)-pinanediol 1-ammonium trifluoroacetate-3-methylbutanyl-1-boronate according to steps 4-5 in Example 2. Add the above-mentioned (1R)-(S)-pinanediol 1-ammonium trifluoroacetate-3-methylbutanyl-1-boronate 303g, N-Boc-L-phenylpropane to a 10-liter reaction vessel Amino acid 212g, TBTU 283g, dichloromethane 3.1kg; control the temperature at about 0°C and add 310g of diisopropylethylamine dropwise for about 0.5 hours, and continue stirring for 0.5 hours after the addition; TLC or HPLC track the reaction to confirm the raw materials ( 1R)-(S)-pinanediol 1-ammonium trifluoroacetate-3-methylbutanyl-1-boronate was converted com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com