Shuttle plasmid and method for efficiently expressing cecropin and lysozyme genes
A shuttle plasmid, high-efficiency expression technology, applied in genetic engineering, plant genetic improvement, chemical instruments and methods, etc., can solve the problems of low expression amount, high cost, and inability to large-scale production of bactericidal peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
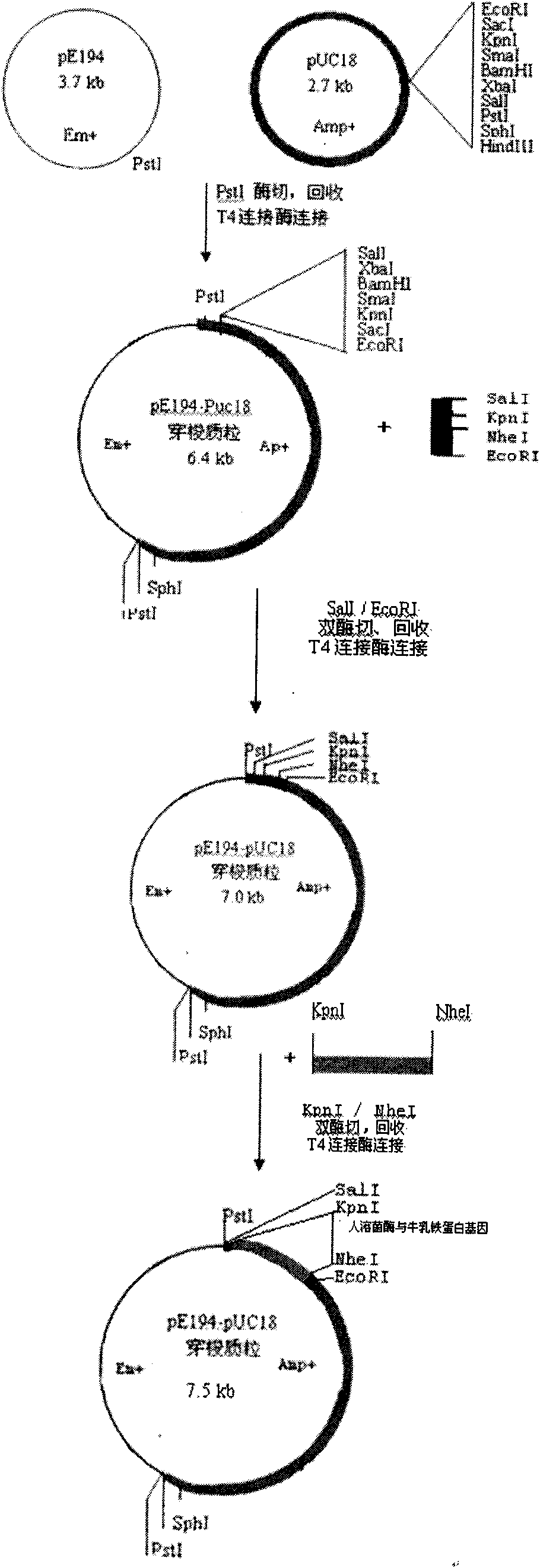
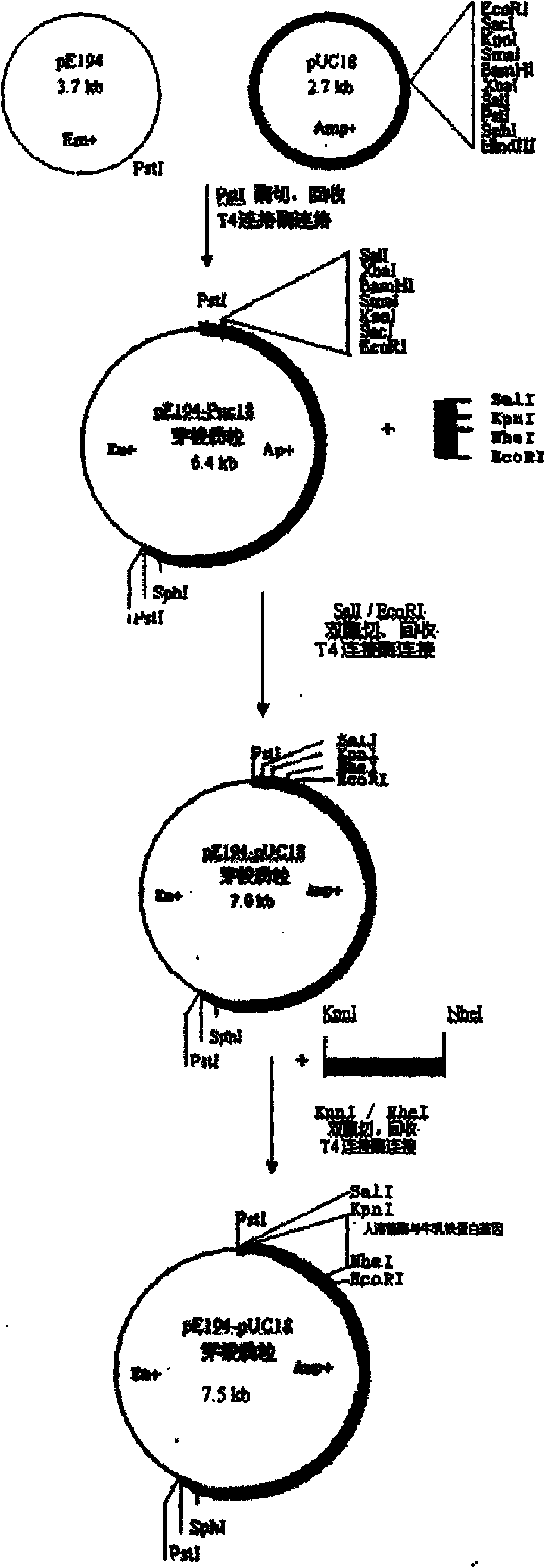
[0040] The construction of Escherichia coli and Bacillus subtilis shuttle plasmids is as follows: figure 1 As shown, the Escherichia coli pUC18 plasmid and the Staphylococcus aureus pE194 plasmid were respectively digested with PstI, and the enzyme digestion system was 20uL: ddH 2 O 7uL, 10xH buffer 2uL, PstI 1uL, pUC18 or pE194 10uL, digestion overnight at 37°C. Enzyme digestion was completed by 1.0% agarose gel electrophoresis, and the corresponding fragments were recovered and purified. Then in 20uL system (ddH 2 O 5.5uL, T4 ligation buffer 2uL, T4 ligase 0.5uL, pUC18 and pE194 each 6uL) for T4 DNA ligase ligation at 16°C overnight, CaCl 2 Transform competent Escherichia coli DE3 by this method, after the transformation solution is absorbed by the solid medium, culture it upside down in a 37°C incubator overnight, transformants grown on the Amp+ plate are transferred and stored, and the plasmid is extracted to identify the size of the increased molecular weight and deter...
Embodiment 2
[0050] The specific construction method of the shuttle plasmid is as in Example 1. According to the human lysozyme gene sequence published by Agric Biol Chem, 1986, 50(3): 713-723, etc., the human lysozyme gene was chemically synthesized, and all codons were replaced with preferred codons suitable for expression in prokaryotes. Human lysozyme gene NheI and KpnI enzyme cutting sites are added to the two ends. Human lysozyme gene and shuttle plasmid were digested with NheI and KpnI. Gene 15uL (or shuttle plasmid 8uL), medium salt 2uL, NheI and KpnI each 0.8uL, make up to a total volume of 20uL with water. Digestion at 37°C for 4 hours, 1% agarose electrophoresis, recovery of gene and vector fragments and purification. The connection system is 20uL (ddH 2 (05.5uL, T4 ligation buffer 2uL, T4 ligase 0.5uL, human lysozyme gene 8uL, vector 4uL), T4 DNA ligase ligation overnight at 16°C on a PCR instrument, CaCl 2 Transform competent Escherichia coli DE3 by using the method. After...
Embodiment 3
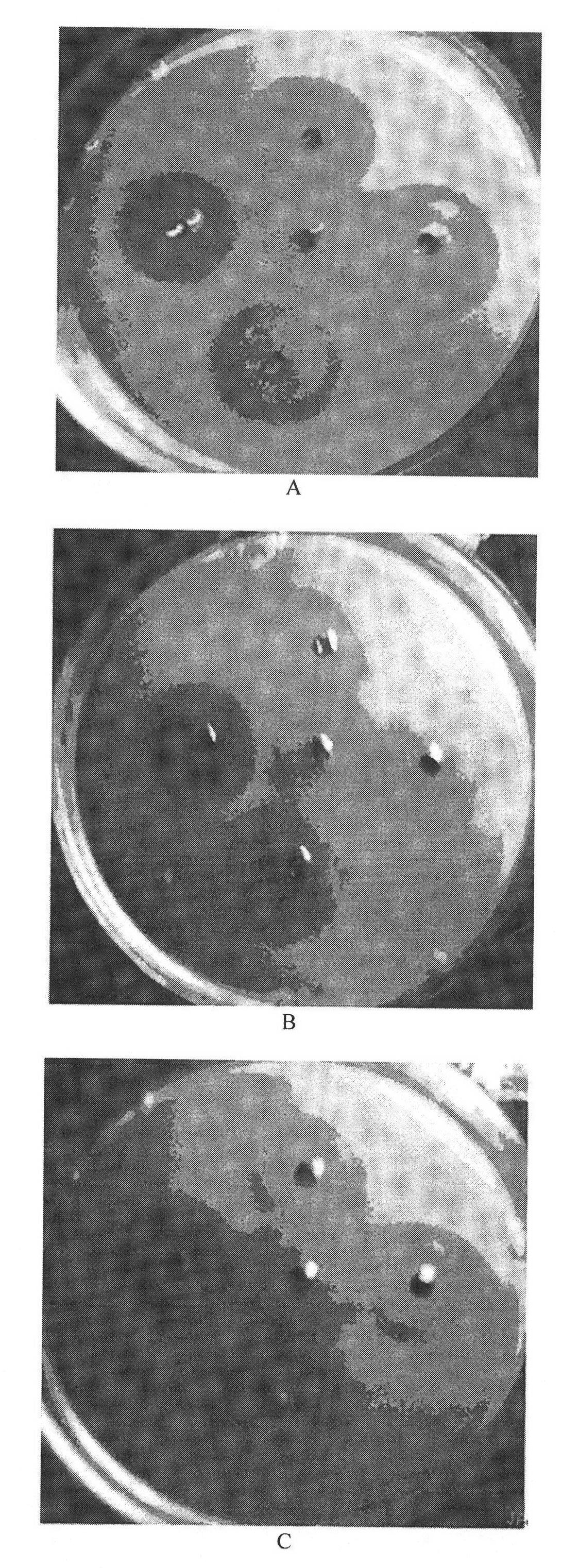
[0055]The specific construction method of the shuttle plasmid is as in Example 1, and the expression, purification, and lysostaphin digestion of the fused shuttle plasmid are as in Example 2. A 15-amino acid peptide segment GlyTrpLysCysArgArgArgTrpGlnTrpArgTrpLys Lys Leu Gly with high-efficiency bactericidal function composed of the 17th to 32nd amino acids of bovine lactoferrin is fused in the human lysozyme gene. The 45 bp sequence of bovine lactoferrin is fused to the C-terminus of the human lysozyme gene DNA molecular sequence, and the 17th amino acid is mutated into Gly, and the 18th and 26th amino acids are mutated into Trp. In order to effectively cut the activated bovine lactoferrin active peptide after the fusion protein is expressed, its sequence is mutated and modified, and the cleavage recognition site sequence of lyso(staphylococcus) enzyme is added, and all amino acids use prokaryote preferred codons. The base sequence of the fusion protein constructed by human l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com