Medicinal composition for treating allergic rhinitis and preparation method and application thereof
A technology of allergic rhinitis and composition, which is applied in the direction of drug combination, pharmaceutical formula, drug delivery, etc., which can solve the problems of rare reports of topical nasal drug use, and achieve the effects of prolonging the drug action time, reducing aggregation, and alleviating allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
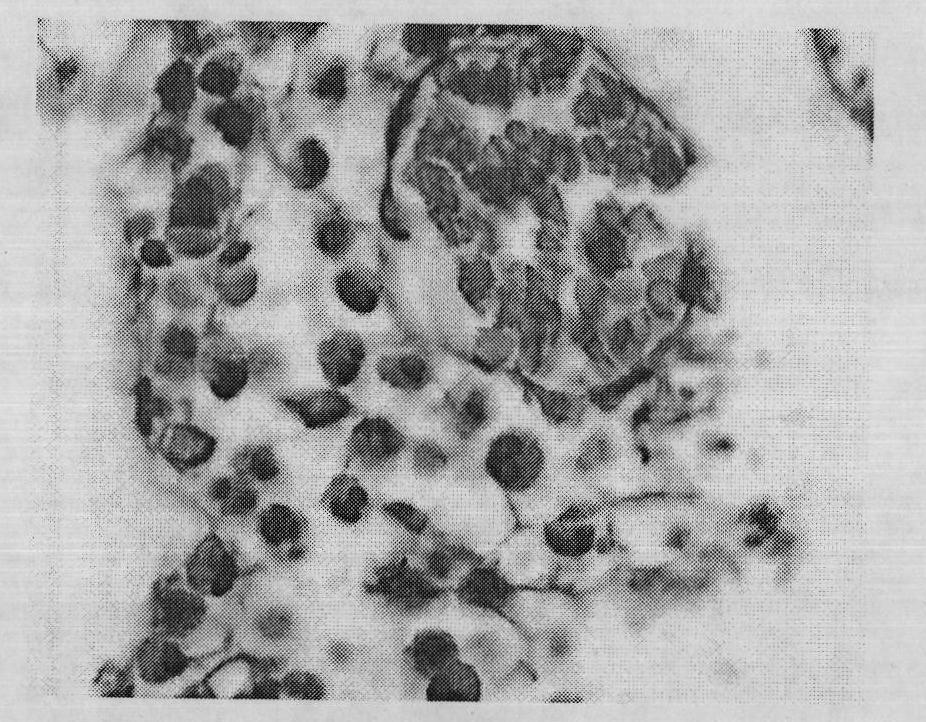
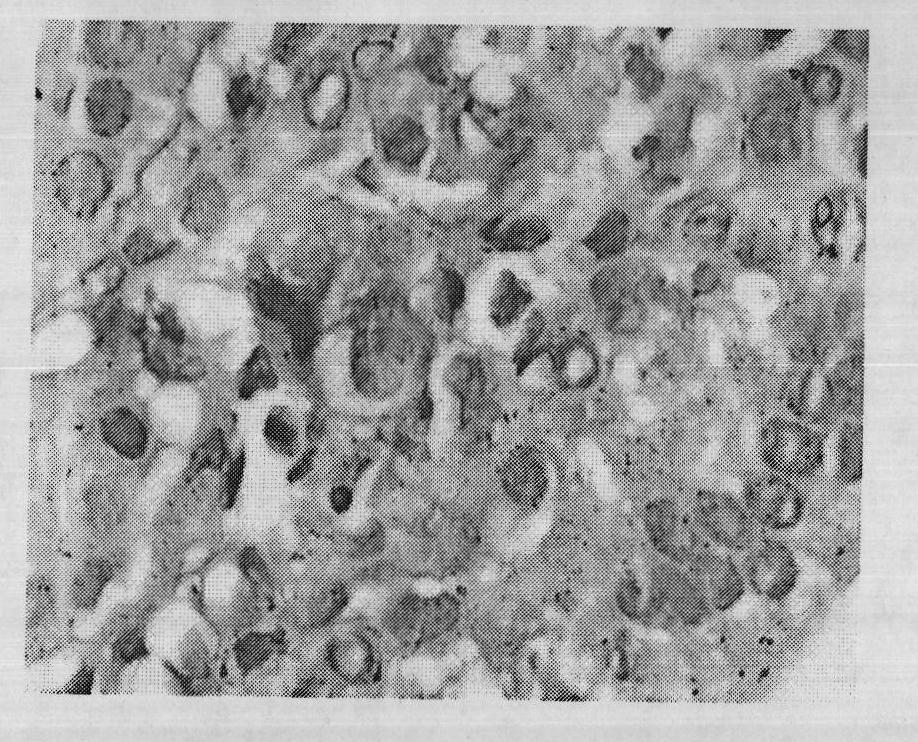
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 pharmaceutical gel drops of the present invention
[0038] a. Take the following raw materials by weight:
[0039] Xu Changqing 20g, cicada slough 4.5g, natural borneol 0.03g, artificial bezoar 0.3g
[0040] b. Take Xu Changqing, add 20 times the amount of water based on the weight of the crude drug, soak for 30 minutes, use steam distillation, extract for 3 hours, collect the distillate, add sodium chloride to refrigerate and crystallize, the volume of the distillate is the same as that of sodium chloride The mass ratio is 100:5, filter, and vacuum dry to obtain paeonol crystals; take paeonol crystals, add appropriate amount of ethanol to dissolve them, and then slowly add them to saturated aqueous solution of β-cyclodextrin, paeonol crystals and β -The mass ratio of cyclodextrin is 1:8, at 45°C, 800r / min stirring inclusion compound for 1 hour, refrigerated for 24 hours, filtered, vacuum-dried to obtain paeonol-cyclodextrin inclusion co...
Embodiment 2
[0046] The preparation of embodiment 2 pharmaceutical gel drops of the present invention
[0047] 1. Extraction process of paeonol in Xu Changqing
[0048] Add 20 times the prescription amount of Xu Changqing and cicada slough with water, soak for 0.5 hours, decoct for 3 hours, collect the distillate, add 5% (g / ml) sodium chloride, refrigerate for 24 hours, filter, wash the crystals with distilled water several times, Vacuum dry at room temperature.
[0049] 2. Paeonol inclusion process
[0050] Take 2g of paeonol and dissolve it in 12ml of ethanol, slowly drop it into 400ml of β-cyclodextrin saturated aqueous solution at a temperature of 45°C, stir for 1h (800r / min), refrigerate for 24h, and filter. A small amount of ethyl acetate was washed 3 times and dried. Vacuum dry at room temperature.
[0051] 3. Borneol inclusion process
[0052] Dissolve 1g of borneol in 10ml, slowly drop into 280ml of β-cyclodextrin saturated aqueous solution, water temperature 30°C, stirring t...
Embodiment 3
[0056] Embodiment 3 Preparation of medicament spray of the present invention
[0057] Get Xu Changqing 14g, cicada slough 5.85g, borneol 0.039g, bezoar 0.39g, extract medicinal materials according to the method described in Example 1, add conventional auxiliary materials to the concentrated solution, put into a spray bottle, and prepare a spray.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com