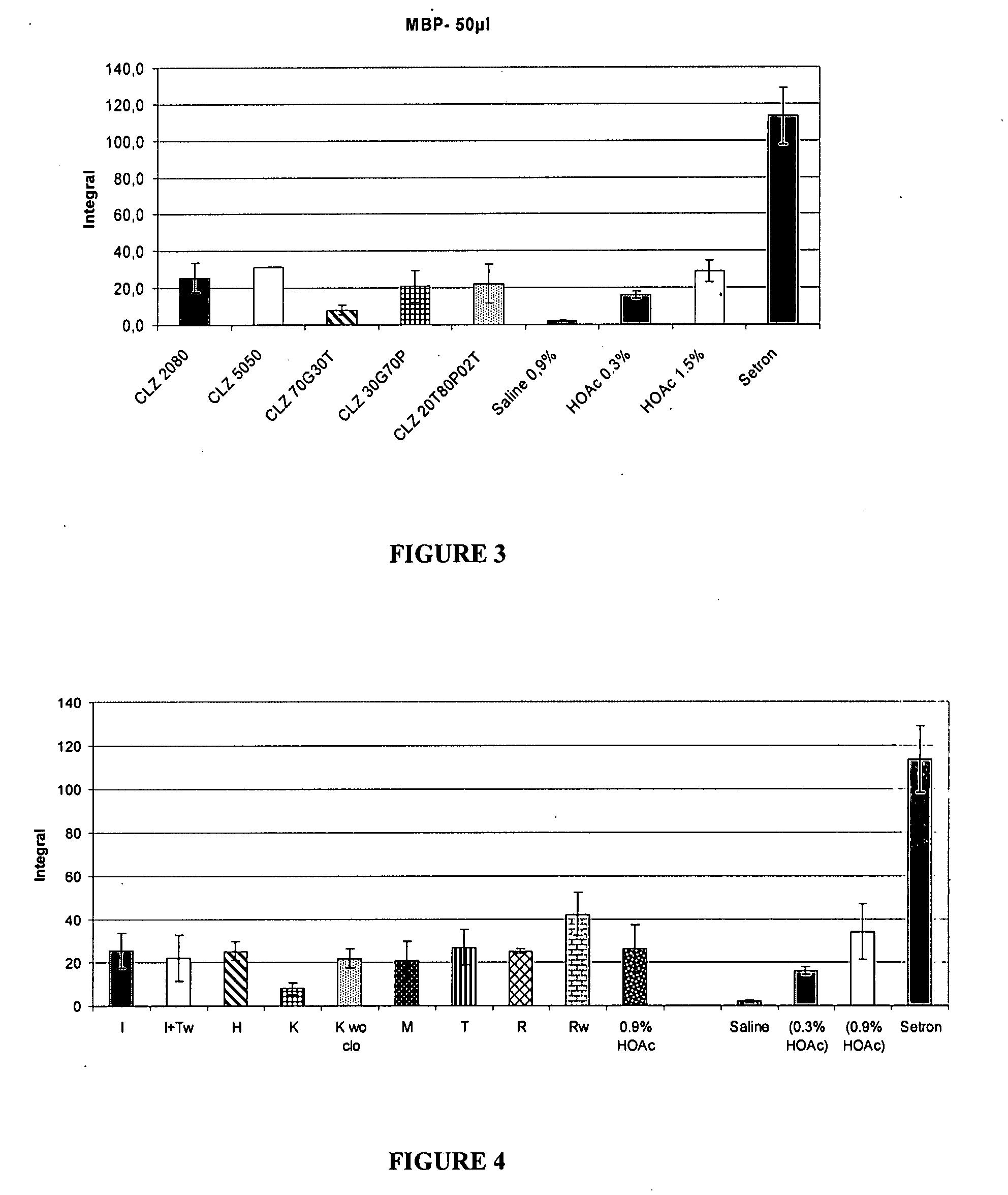
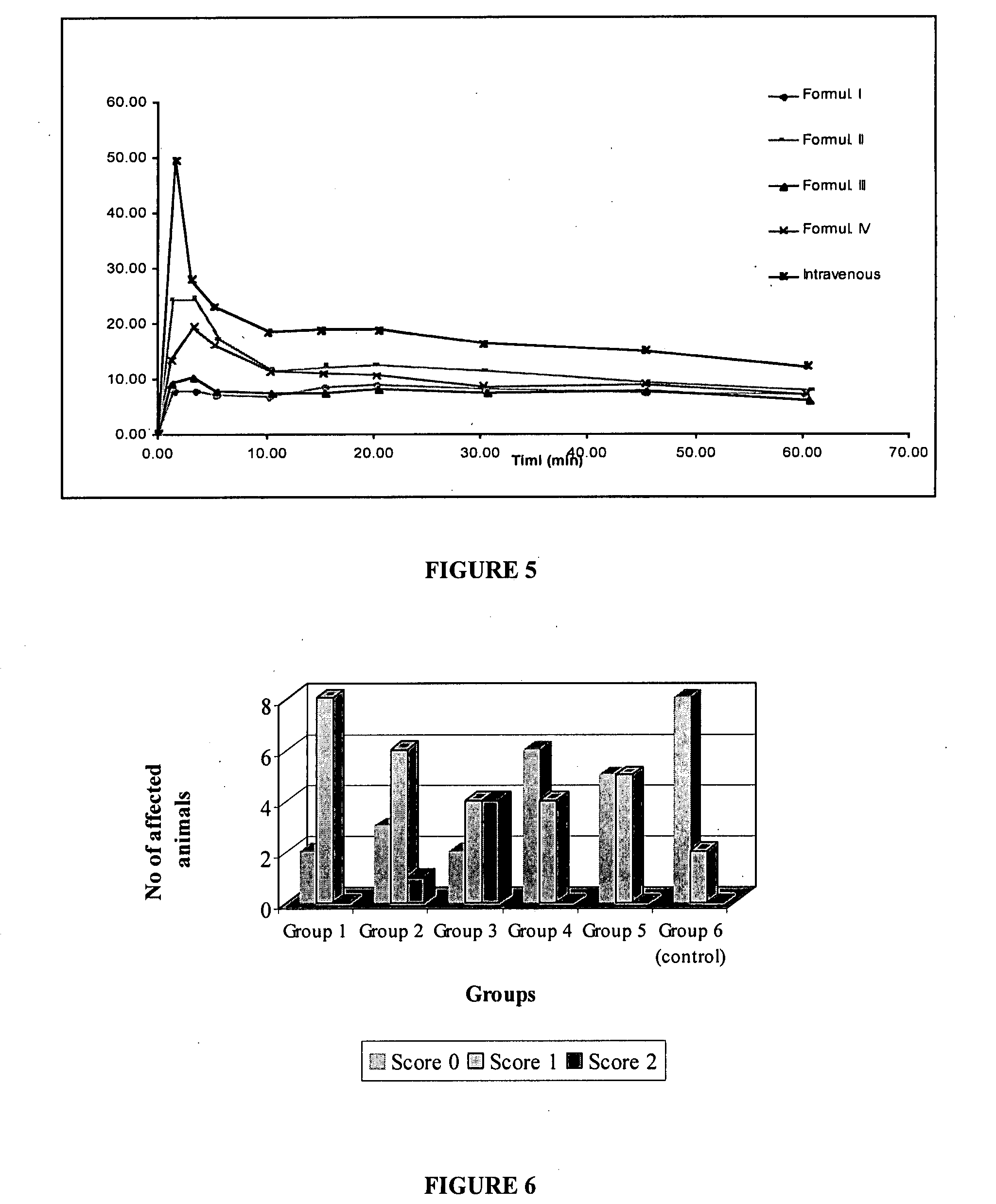
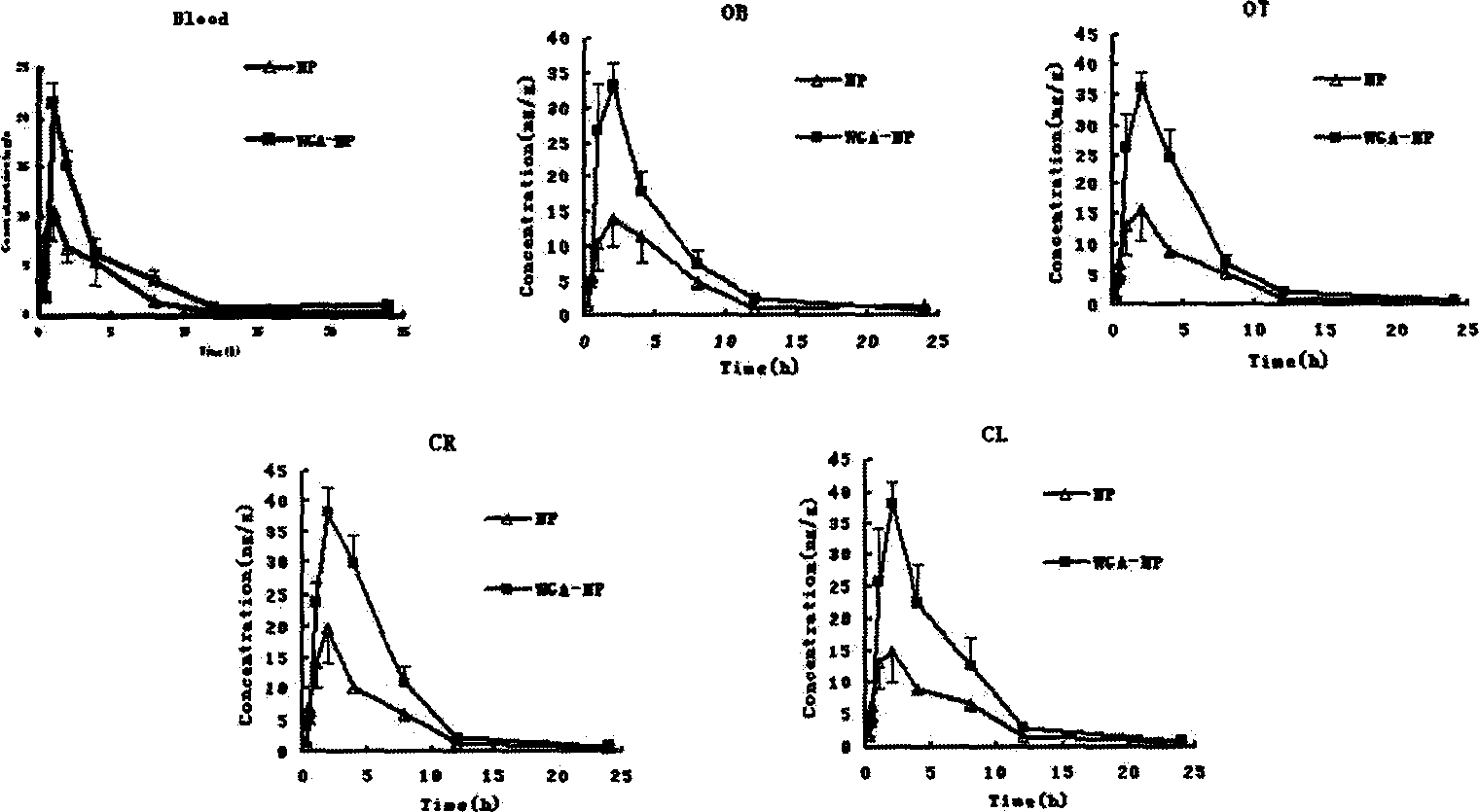
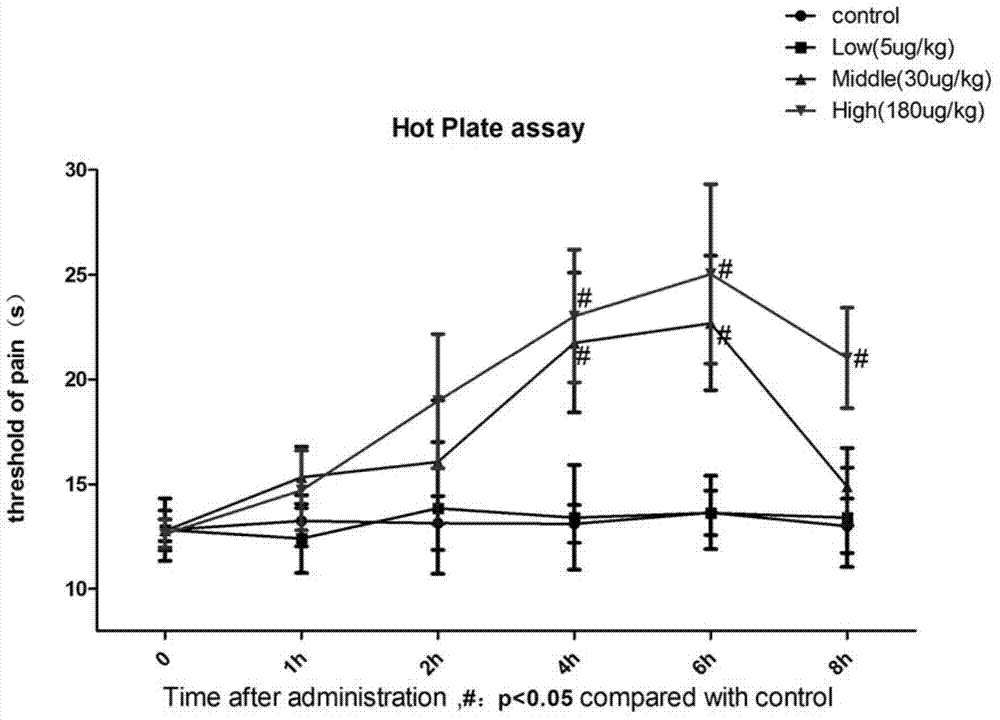
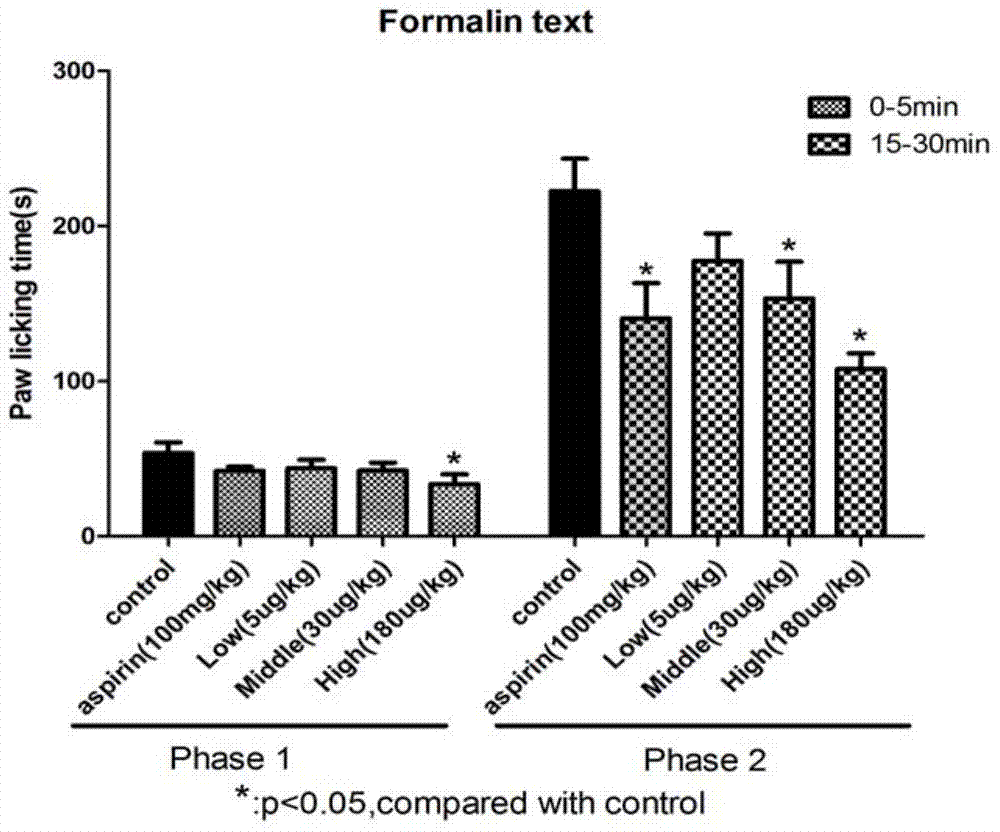
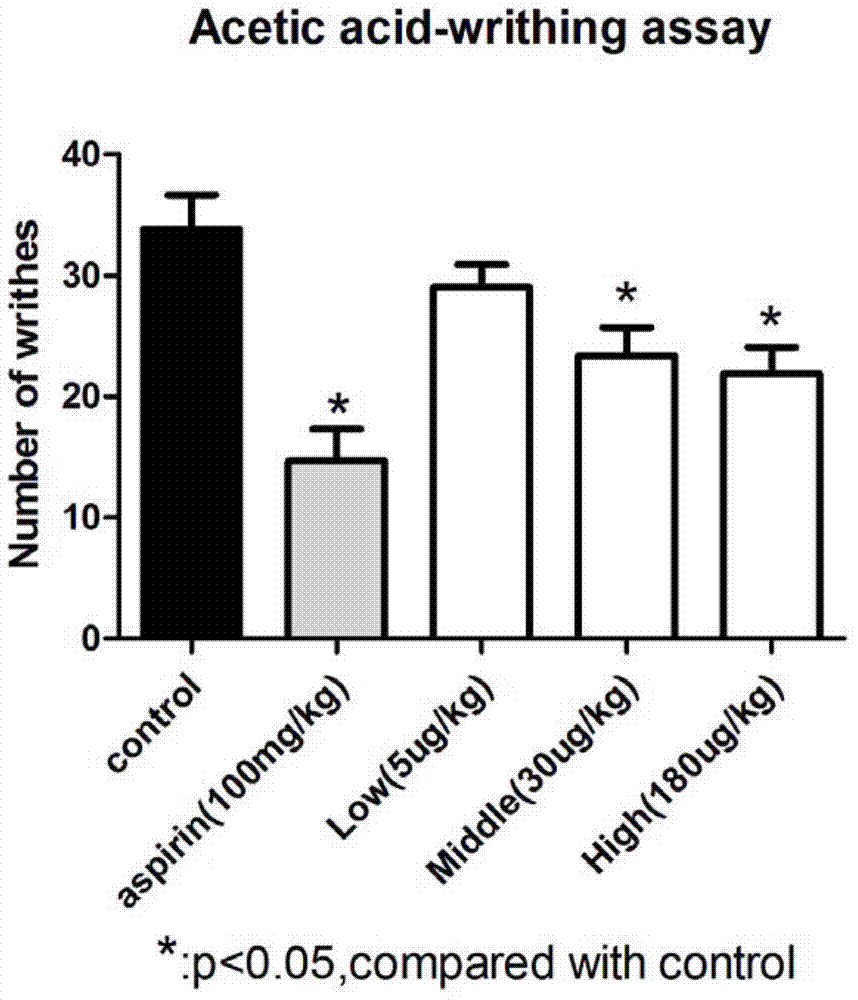
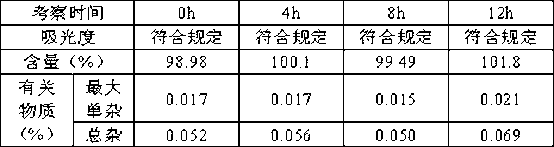
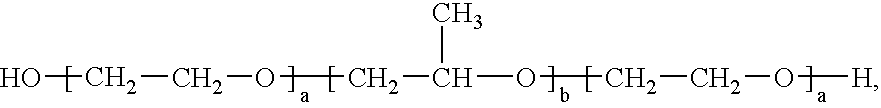Patents
Literature
134 results about "Mucous membrane of nose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
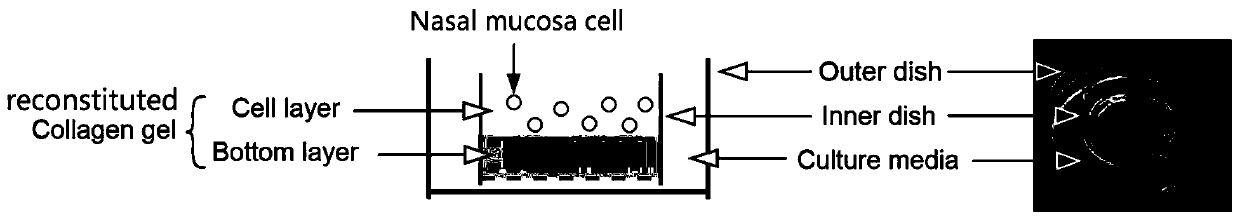
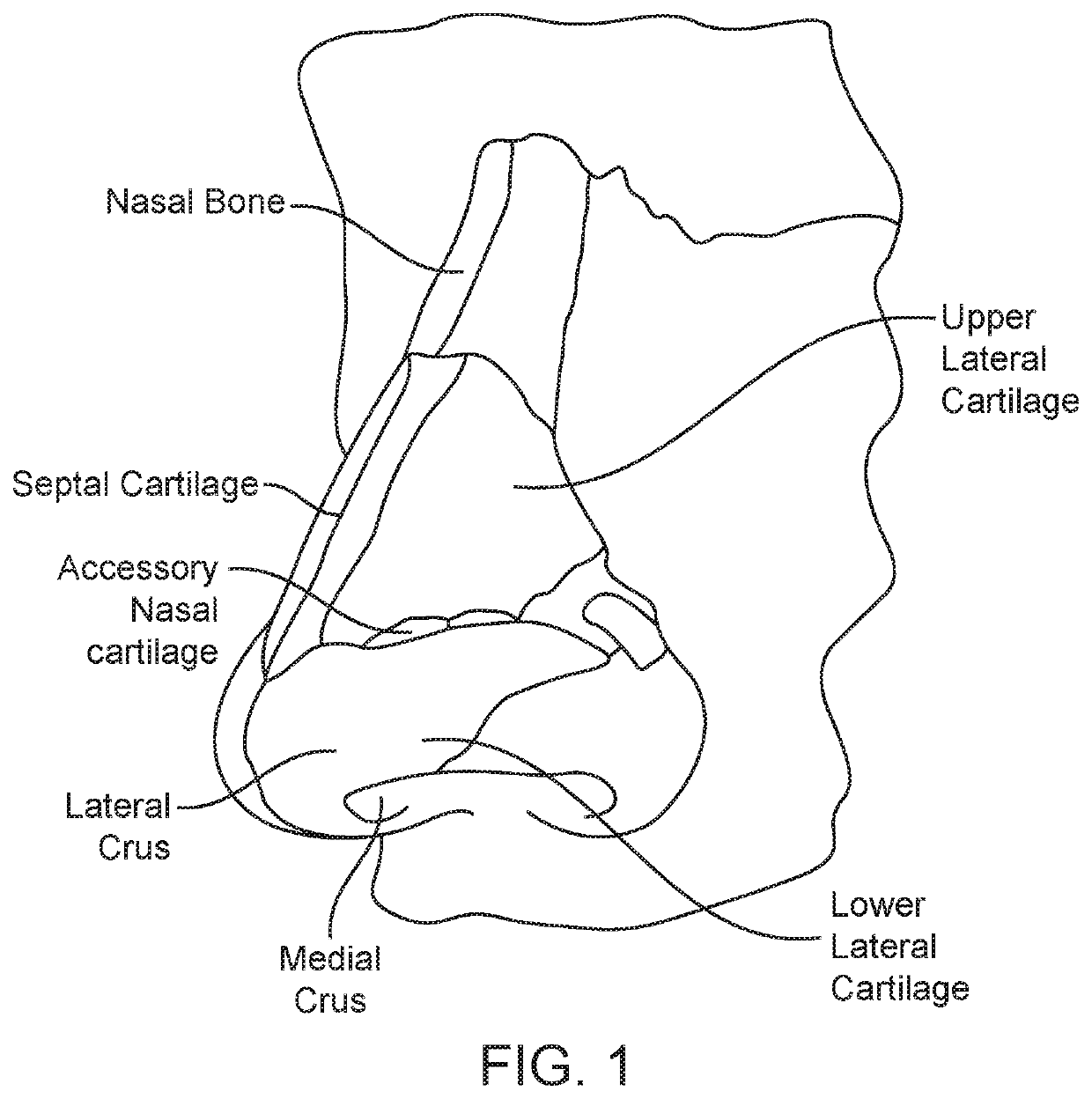
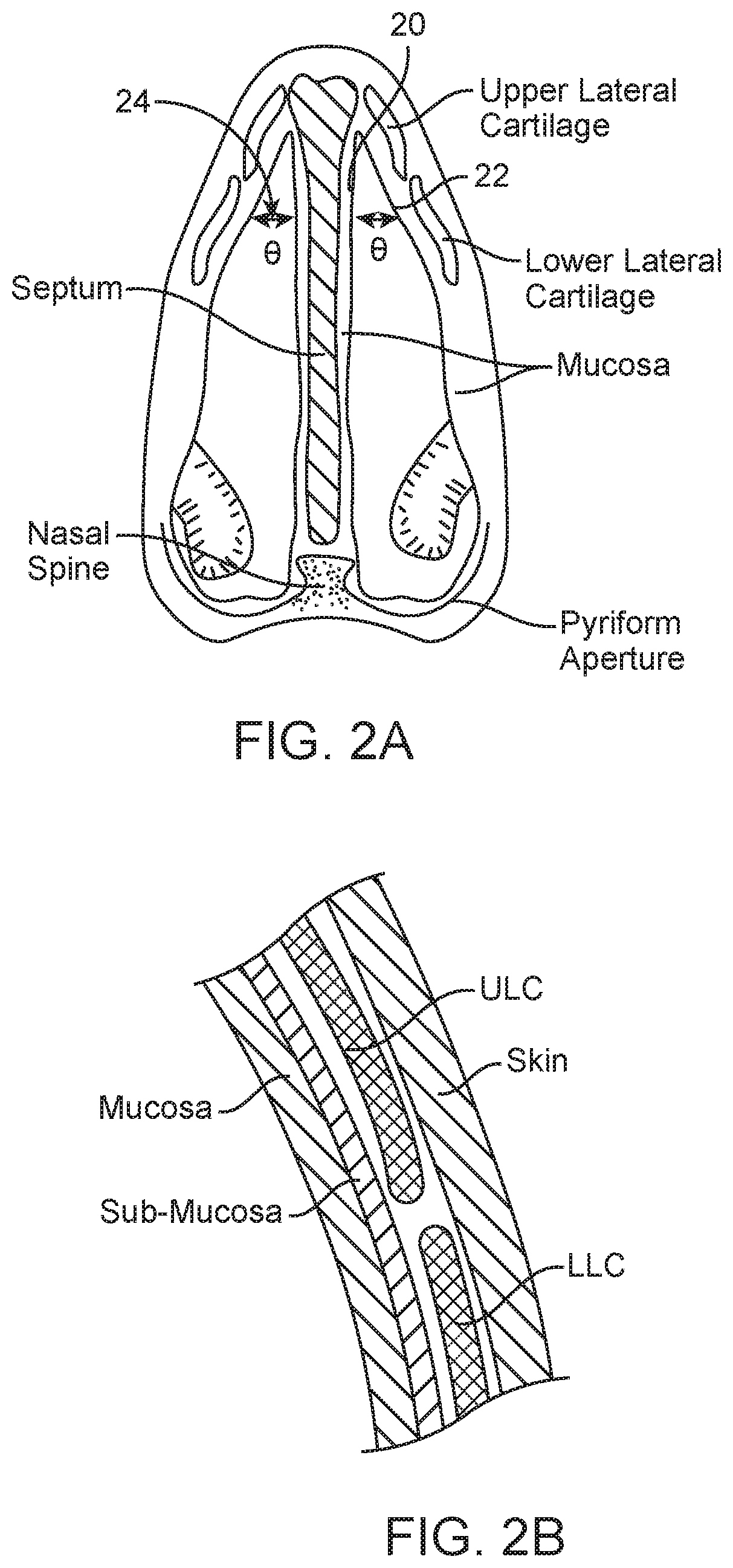
The nasal mucosa lines the nasal cavity. It is part of the respiratory mucosa, the mucous membrane lining the respiratory tract. The nasal mucosa is intimately adherent to the periosteum or perichondrium of the nasal conchae. It is continuous with the skin through the nostrils, and with the mucous membrane of the nasal part of the pharynx through the choanae. From the nasal cavity its continuity with the conjunctiva may be traced, through the nasolacrimal and lacrimal ducts; and with the frontal, ethmoidal, sphenoidal, and maxillary sinuses, through the several openings in the nasal meatuses. The mucous membrane is thickest, and most vascular, over the nasal conchae. It is also thick over the nasal septum where increased numbers of goblet cells produce a greater amount of nasal mucus. It is very thin in the meatuses on the floor of the nasal cavities, and in the various sinuses. It is one of the most commonly infected tissues in adults and children. Inflammation of this tissue may cause significant impairment of daily activities, with symptoms such as stuffy nose, headache, mouth breathing, etc.
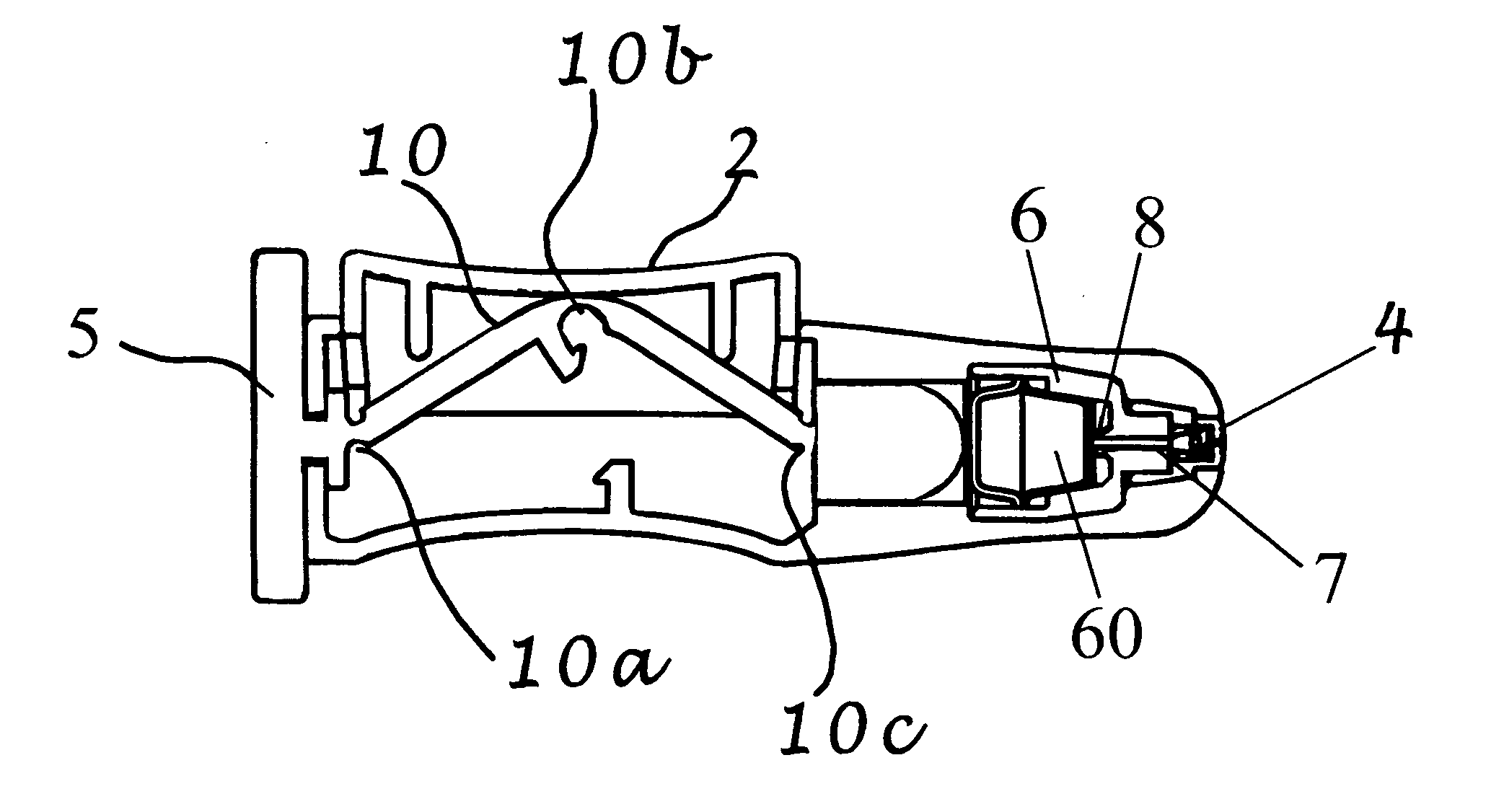
Intranasal Cartridge Devices
ActiveUS20080177246A1Precise alignmentProvide stabilityAmpoule syringesJet injection syringesNasal passageNasal passages
Intranasal delivery devices include dosage forms containing medical compositions for use in the intranasal devices, and methods of delivering medical compositions to the nasal mucosa of users. The devices dispense a predetermined quantity of fluid into the nasal passage of a user, in which the predetermined quantity of fluid is contained in, or produced in a dosage form or blister that is crushed by a plunger with sufficient force to drive the dosage form against a piercing mechanism, piercing the dosage form and forcing the liquid contents from the dosage form and through a delivery channel into a spray to be directed into the nasal passage of a user. The plunger is connected to a trigger device by a linkage that confers a mechanical advantage to the trigger mechanism.
Owner:MYSTIC PHARMA INC
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833550B2Reduce morbidityNot impart bitter tastePowder deliverySenses disorderNasal cavityAzelastine
Dry powder formulations of drugs such as antihistamine for nasal administration are provided where the drug is retained in the nasal cavity, and systemic side effects minimized or eliminated, through the selection of a narrow particle size range, between approximately 10 and 20 microns in diameter. In a preferred embodiment wherein the drug is an antihistamine, retention of the antihistamine at the nasal mucosa is improved and the bitter aftertaste associated with liquid antihistamine formulations significantly reduced. By making a dry powder formulation of an antihistamine (e.g., azelastine) having an average particle size of between 10 and 20 microns, the antihistamine is restricted primarily to the desired target organ, the nasal mucosa. Because the active ingredient stays in the nasal region, a lower dose can be used to achieve the same desired effect. As demonstrated by the examples, this lower dose reduces the incidence of somnolence, and because the active ingredient remains at the target organ and does not accumulate in the back of the throat and mouth, this formulation does not impart a bitter taste.
Owner:MANNKIND CORP
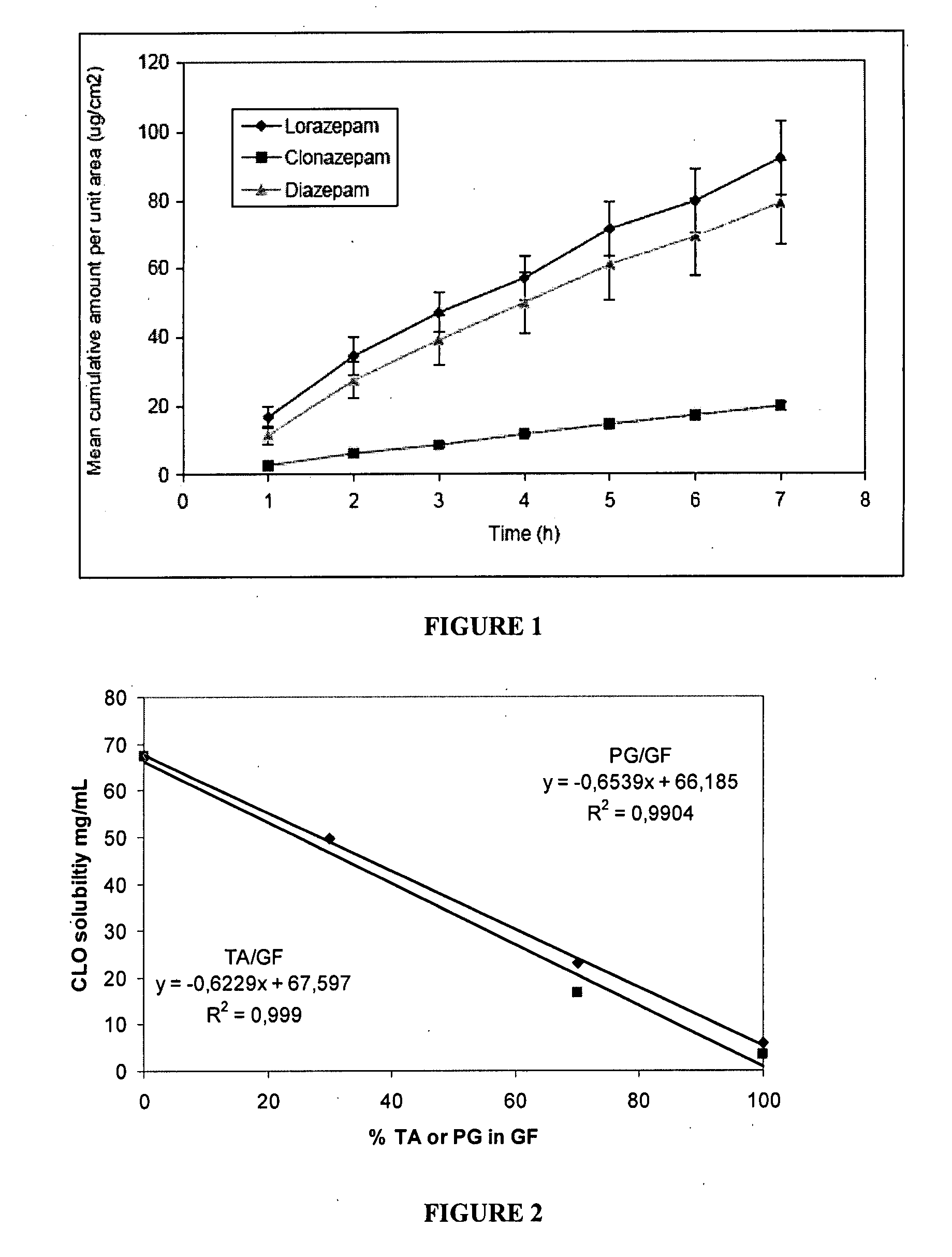
Pharmaceutical compositions of benzodiazepines and method of use thereof
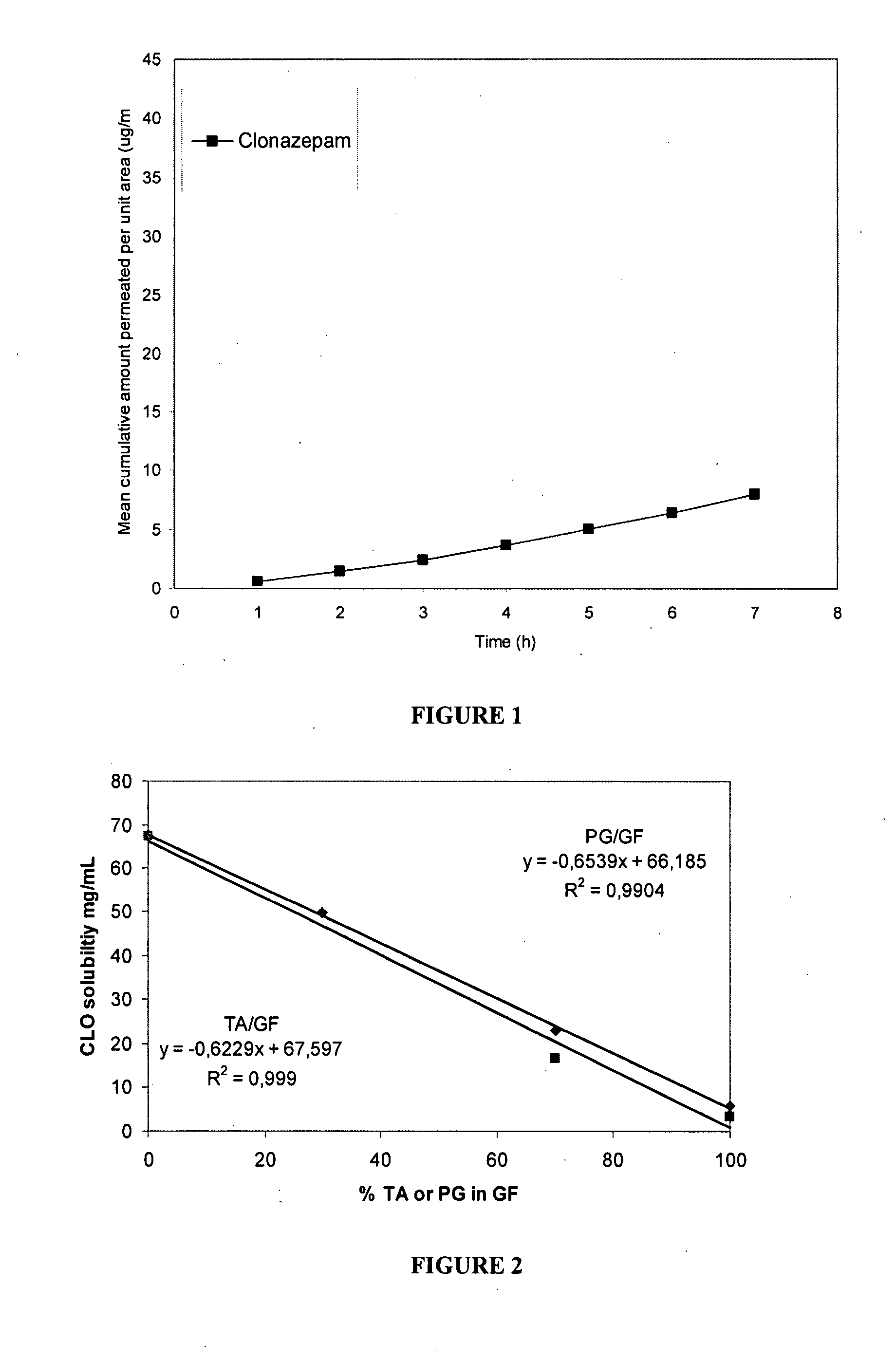
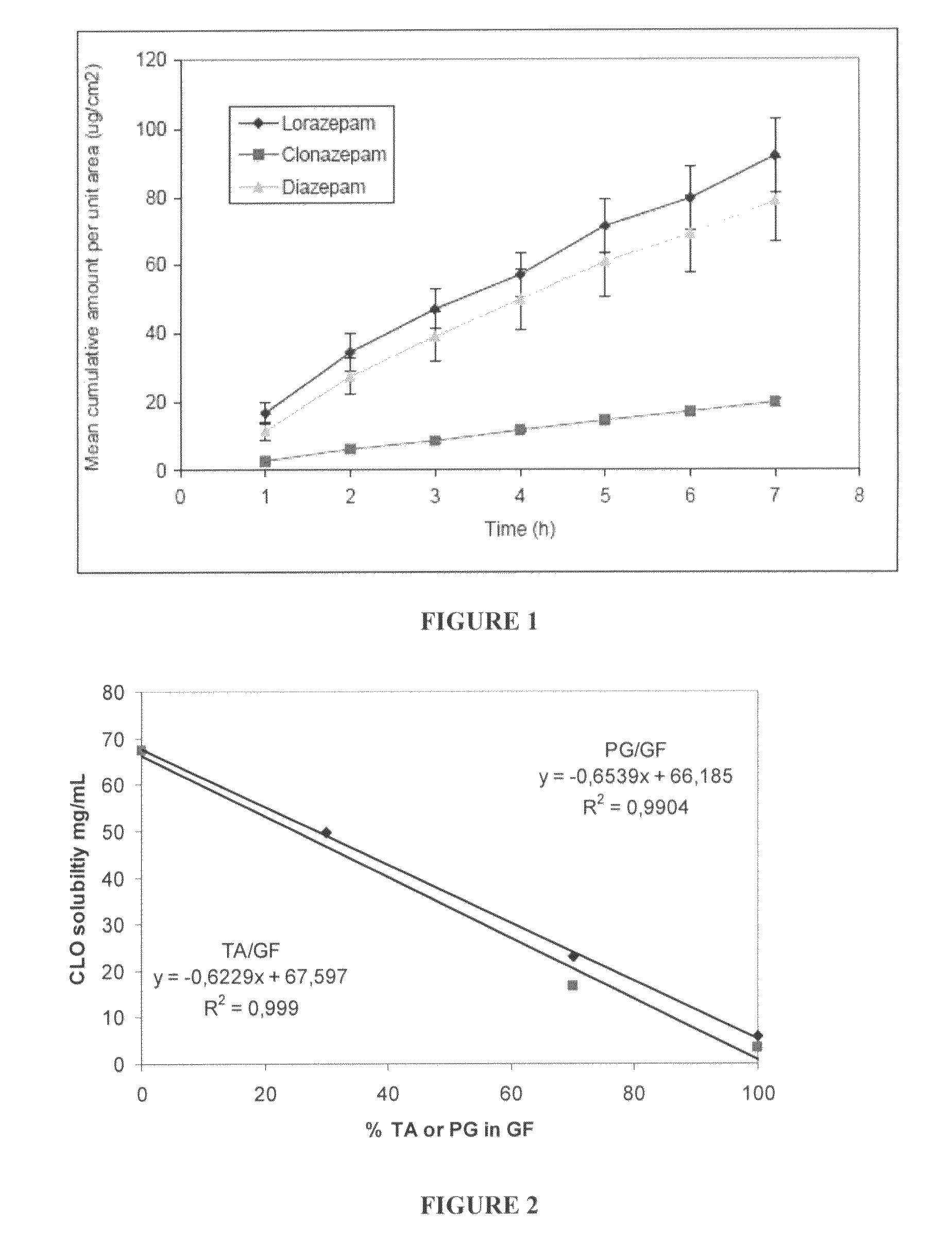
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833549B2Reduce morbidityNot impart bitter tasteBiocidePowder deliveryNasal cavityAzelastine
Owner:MANNKIND CORP
Nasal peptide pharmaceutical formulation
InactiveUS20050158247A1Optimal therapeutic dose levelConstant ratePeptide/protein ingredientsAerosol deliveryEpitheliumDiluent
The invention provides a pharmaceutical formulation comprising: (1) THAM, which is tris(hydroxymethyl)aminomethane, as a selective absorbefacient to enhance through the nasal mucosa-lined epithelium the absorption of substances of peptide nature; and (2) a therapeutically effective amount of active nasal peptide, its pharmaceutically acceptable salt or its peptidic fragment; in a pharmaceutically acceptable, aqueous liquid diluent or carrier, said formulation being in a form suitable for nasal administration.
Owner:THERAPICON SRL
Agglutinin-modified drug delivery system from nose to brain
InactiveCN1839799ASmall toxicityImprove central preventionPowder deliveryNervous disorderNasal cavitySide effect
This invention belongs to chemistry pharmaceutical field and relates to a medication transfer system, especially relates to an agglutinin masked medication-load transfer mechanism transferring from nose to brain. This invention comprises medication carrier nanograin, vesicle or lipid body surface finish agglutinin. By using the transfer system, resort time of medication-load system on nasal mucosa can be prolonged, dielectric mucosa absorb the medication-load system and selectively deliver small molecular chemistry medication, diagnosis medication, polypeptide proteins and gene medication into brain. This invention can deliver more medication into brain and relatively decrease medication in outer tissue, so it can depress toxic action at every pore while toning up prevention, cure and diagnosis effect of backbone diseases.
Owner:FUDAN UNIV
Pharmaceutical compositions of clonazepam and method of use thereof
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA INC
Compositions and methods for treating seizures
Owner:DRAGTEK CORP
Pharmaceutical Combination for the Treatment of Cns Functional Disorders
InactiveUS20080207600A1Less side effectsImprove permeabilityBiocideNervous disorderTherapeutic effectUnexpected therapeutic effect
The invention relates to a method and a pharmaceutical combination for the treatment of CNS disorders, which includes at least a first compound having therapeutic effect on the CNS, and a second compound which facilitates penetration through the hematoencephalic barrier of the former. The second compound is administered endonasally, and has refectory (mainly neuro- and vasoactive) effects over structures and receptors of nasal mucous membrane, mainly receptors of vomeronasal systems and trifacial nerve.
Owner:OBSCHESTVO OGRANICHENNOI OTVETABTVENNOCTIYU PARKINFARM
Antigen covalently bound chitosan nanoparticle-based nasal immune carrier
ActiveCN104274830ANot easy to fall offOvercoming the disadvantages of encapsulating antigensCarrier-bound antigen/hapten ingredientsPharmaceutical non-active ingredientsTumor-Related ProteinDisease
The invention belongs to the technical field of medicines, and relates to an antigen covalently bound chitosan nanoparticle-based nasal immune carrier and a preparation method thereof. The carrier is composed of a nanoparticle and an antigen, the antigen is covalently bound to the surface of the nanoparticle, and the nanoparticle is prepared by adopting a raw material chitosan and its derivatives; and the antigen is selected from from infectious diseases or tumors related proteins or polypeptides. The carrier can significantly improve the neck lymph node targeting of people nasally dosed with the antigen proteins or polypeptides, enhances the mucosal and systemic immune response, has the advantages of simple preparation, stable process, high antigen utilization rate, difficult antigen shedding, benefiting of the improvement of the cervical lymph node transportation of the antigens, good nasal immune effect and no nasal mucosa toxicity, and is suitable for preventing respiratory infectious diseases.
Owner:FUDAN UNIV
Pharmaceutical compositions of benzodiazepines and method of use thereof
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA INC
Nasal mucosa organoid culture medium and culture method
ActiveCN111117946AProliferateCapable of multi-lineage differentiationCulture processEpidermal cells/skin cellsEpitheliumGoblet cell
The invention discloses a culture medium and culture method of a nasal mucosa tissue. According to the culture medium and culture method of the nasal mucosa tissue, epithelial-mesenchymal / matrix components are integrated in an organoid culture system based on a gas-liquid interface method for the first time, and by inducing proliferation and differentiation of adult stem cells in a fresh nasal mucosa epithelial tissue, an organoid which is composed of multiple kinds of cells, including ciliated cells, goblet cells, club cells and basal cells, and is close to an internal mucosa in structure andfunction can be obtained, and becomes an in-vitro nasal mucosa model with proliferation and multi-lineage differentiation capability.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Methods and devices to treat nasal airways
ActiveUS10722282B2Easy to breatheReduce airflow resistanceElectrotherapySurgical needlesNasal foramenBiomedical engineering
Owner:AERIN MEDICAL INC
Application of maidenhair volatile oil in rhinitis resistance medicine
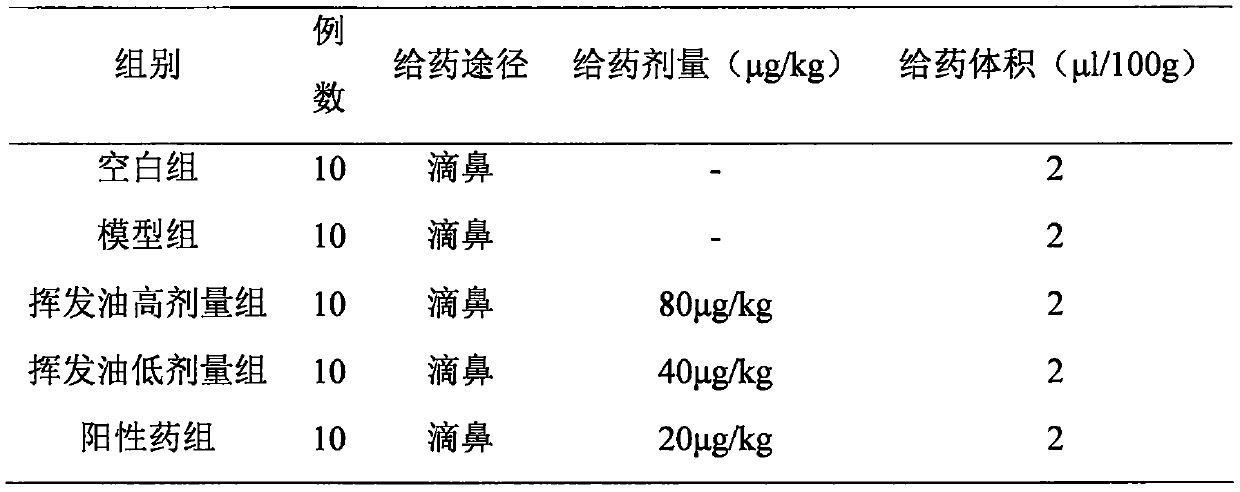
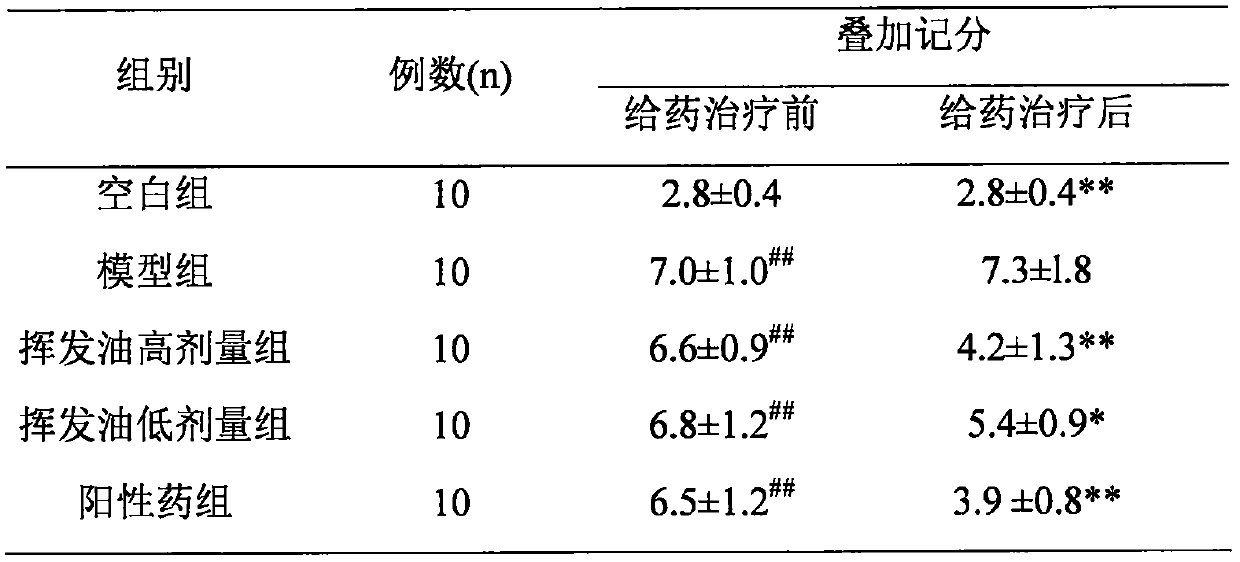
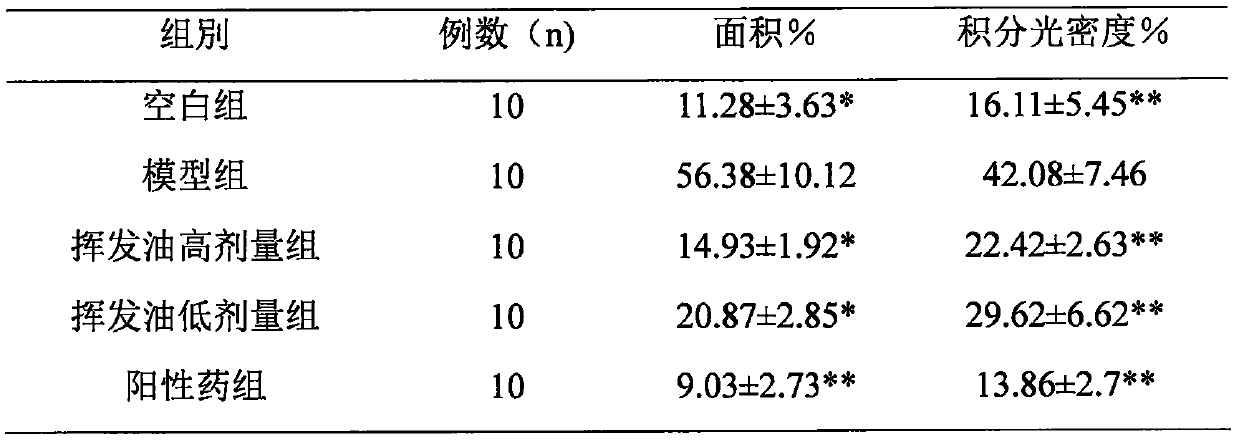
The invention relates to application of maidenhair volatile oil in a medicine for treating rhinitis. A method comprises the step of grinding a whole herb of maidenhair and then extracting the maidenhair by a steam distillation method or a carbon dioxide supercritical fluid extraction method to form the volatile oil. No toxic reagent is used by the two extraction methods, and the extracted volatile oil has the characteristics of no toxicity, no residue, simple extraction technology and high extraction efficiency. A medicinal auxiliary material can be added into the volatile oil to prepare a nasal drop or a spray as the medicine for treating the rhinitis. Pharmacological experiments show that the volatile oil has a better pharmacological action on an allergic rhinitis model of a guinea pig, can obviously alleviate symptoms of a nose of the allergic rhinitis model of the guinea pig, reduces an ethological accumulative integral, and significantly relieves edema of a nasal mucosa of the allergic rhinitis model of the guinea pig.
Owner:蔡德成 +2
Nasal cavity channel diameter detection tube
InactiveCN105455817AAvoid damageElasticBalloon catheterMedical devicesAspiratorNasal Cavity Epithelium
The invention discloses a nasal cavity channel diameter detection tube and relates to the field of medical consumables. The detection tube is provided with a detection tube body, wherein the front end of the detection tube body is provided with a guide part which is small in front and big in back, and the outer side of the detection tube body is provided with a length mark; the upper portion and the lower portion of the tube wall of the guide part are provided with through holes, a measuring air bag is arranged outside the portion, behind the guide part, of the detection tube body and connected with an inflator through an inflatable pipe via a one-way valve, and the inflator is provided with a scale which corresponds to the diameter of the measuring air bag and reflects the air injection amount; the detection tube body is connected with a breathing machine or an aspirator through an inlet and can provide an oxygen supply channel or suck secreta in the nasal cavity. The detection tube can achieve measurement of the passing diameter of the nasal cavity channel to provide a basis for choosing a proper cannula or catheter for a nasal trachea cannula and avoid aspiration by mistake caused by nasal mucosa damage and bleeding when an improper cannula or catheter is adopted.
Owner:LIUZHOU PEOPLES HOSPITAL +1
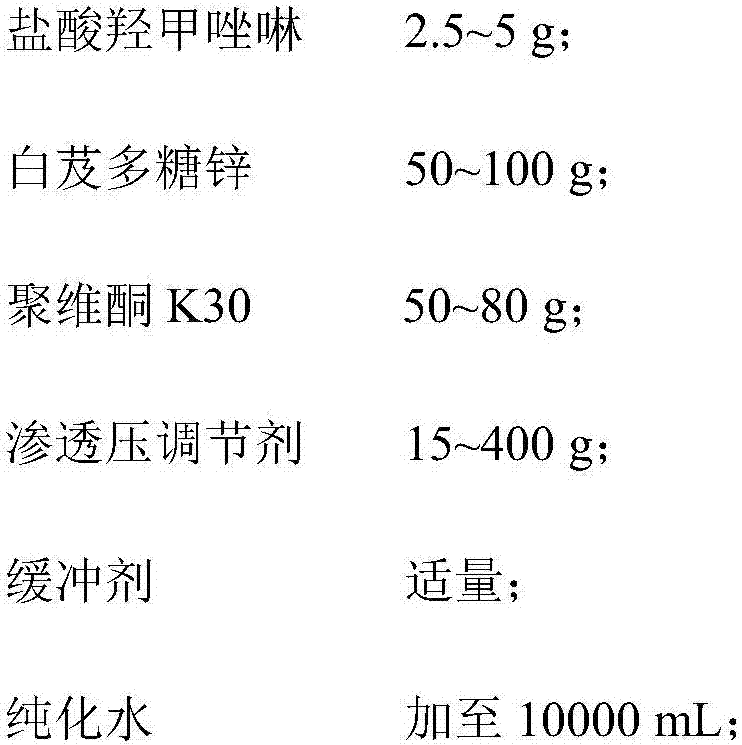
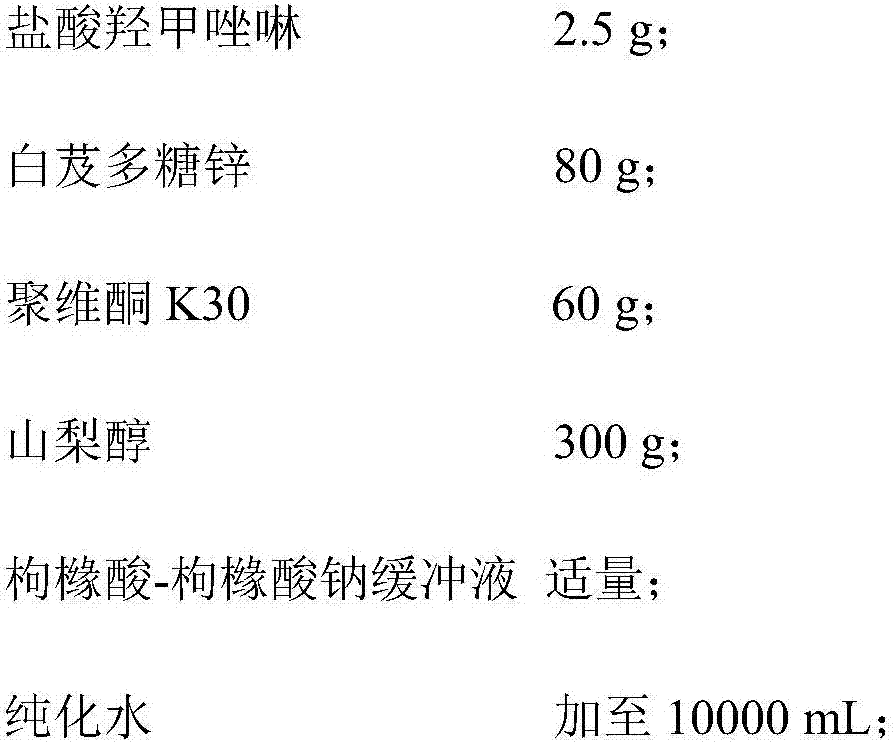
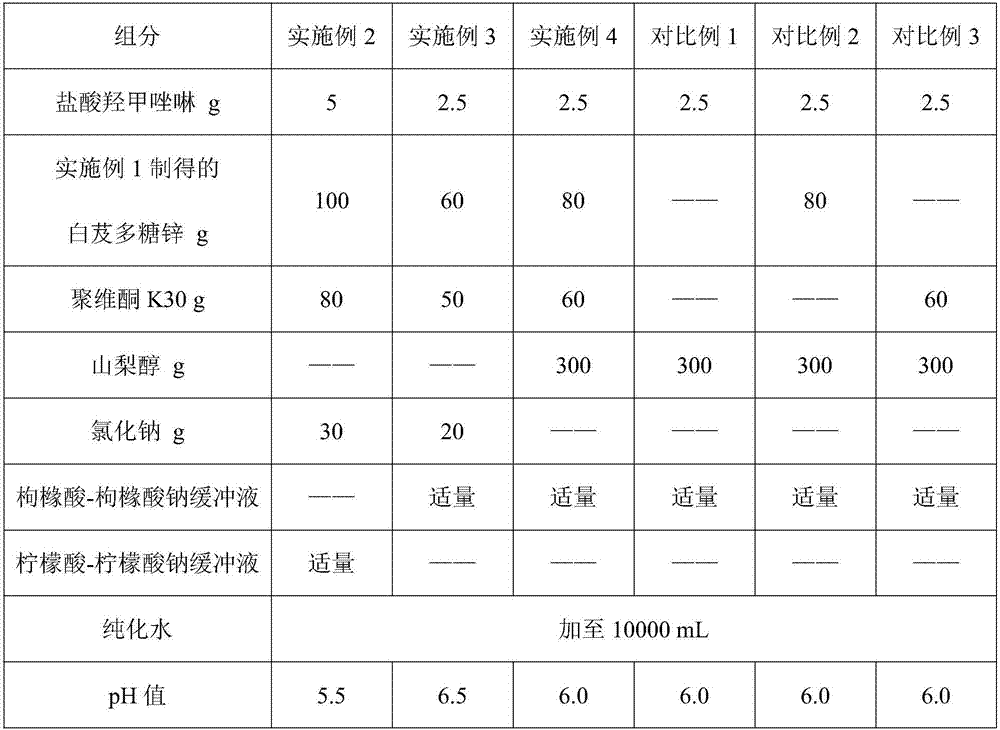
Oxymetazoline hydrochloride nasal spray and preparation method thereof
ActiveCN107362141AImprove stabilityLow nasal irritationOrganic active ingredientsAerosol deliveryIrritationDrug
The invention belongs to the technical field of medicines, and provides an oxymetazoline hydrochloride nasal spray and a preparation method thereof. The nasal spray comprises the following components by weight: 2.5-5g of oxymetazoline hydrochloride, 50-100g of blettila striata polysaccharide zinc, 50-80g of povidone K30, 15-400g of an osmotic pressure regulating agent, a proper amount of a buffer agent, and the balance of purified water till 10,000 mL. The pH value of the nasal spray provided by the invention is 5.5-6.5. Due to mutual action of the components of the oxymetazoline hydrochloride nasal spray provided by the invention, the stability of medicinal active component, namely the oxymetazoline hydrochloride, but also the stability a medicinal preparation are improved, and meanwhile the nasal mucosa irritation of the preparation is alleviated.
Owner:深圳大佛药业股份有限公司
Nasal cavity washing liquid for treating rhinitis
InactiveCN106619969APromotes self-healingPromote healingOrganic active ingredientsPharmaceutical delivery mechanismNasal cavityFlos chrysanthemi
The invention discloses nasal cavity washing liquid for treating rhinitis. The nasal cavity washing liquid comprises the following components in parts by mass: 50-100 parts of a traditional Chinese medicine extraction solution, 0.1-0.2 part of medium-molecular sodium hyaluronate, 0.1-0.2 part of micromolecular sodium hyaluronate, 8.5-9.5 parts of sodium chloride and 850-900 parts of distilled water, wherein the traditional Chinese medicine extraction solution is prepared from a traditional Chinese medicine composition. The traditional Chinese medicine composition comprises the following components: 100-150 parts of boat-fruited scaphium seeds, 150-220 parts of flos chrysanthemi, 20-60 parts of herba houttuyniae, 40-80 parts of radix bupleuri and 40-100 parts of herba menthae. The washing liquid is capable of cleaning the nasal cavity and is also capable of accelerating recovery of damaged nasal mucosa and rapidly curing rhinitis fundamentally.
Owner:四川香龙丹医药科技有限公司
Liquid preparation for rhinitis/allergic rhinitis as well as preparation method and application thereof
ActiveCN103271993AImprove complianceSimple processRespiratory disorderImmunological disordersGLYCYRRHIZA EXTRACTPharmaceutical Substances
The invention relates to the technical field of liquid traditional Chinese medicine preparations and provides a liquid preparation for rhinitis / allergic rhinitis as well as a preparation method and application thereof. The liquid preparation is obtained by virtue of the following method: carrying out centrifugation after dissolving Artemisia rupetris extracts in a solubilizer solution, taking the supernatant, adding Scutellaria baicalensis extracts to the supernatant and completely dissolving the Scutellaria baicalensis extracts; then adding a thickener solution to the solution, mixing the solutions uniformly, then adding liquorice extracts to the mixture, stirring the mixture to dissolve the liquorice extracts, then adding a preservative to the solution, and fully stirring the solution to dissolve the preservative; and finally adding water for injection to the solution and mixing the substances uniformly, thus obtaining the liquid preparation for rhinitis / allergic rhinitis. The liquid preparation is simple in process, has stable and controllable quality, obvious curative effects and no toxic or side effect, directly acts on the surface of nasal mucosa, has high bioavailability, takes effect quickly, is convenient to use and has good patient compliance.
Owner:XINJIANG INST OF MATERIA MEDICA
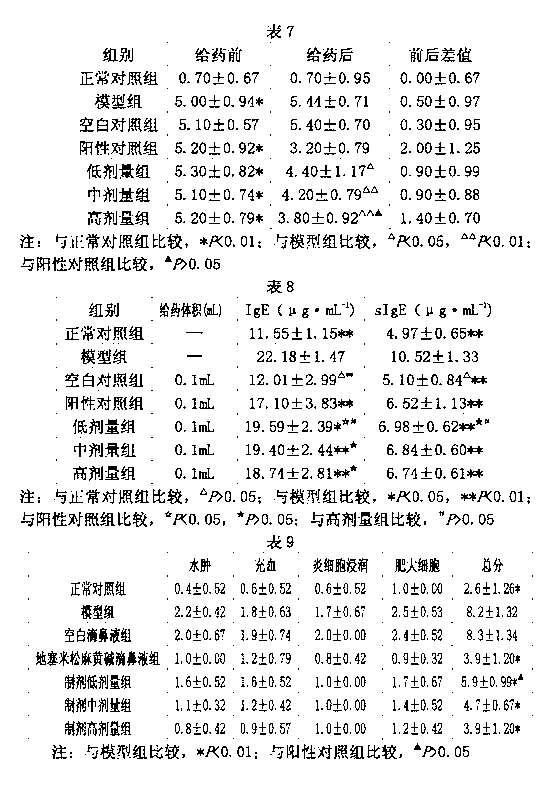
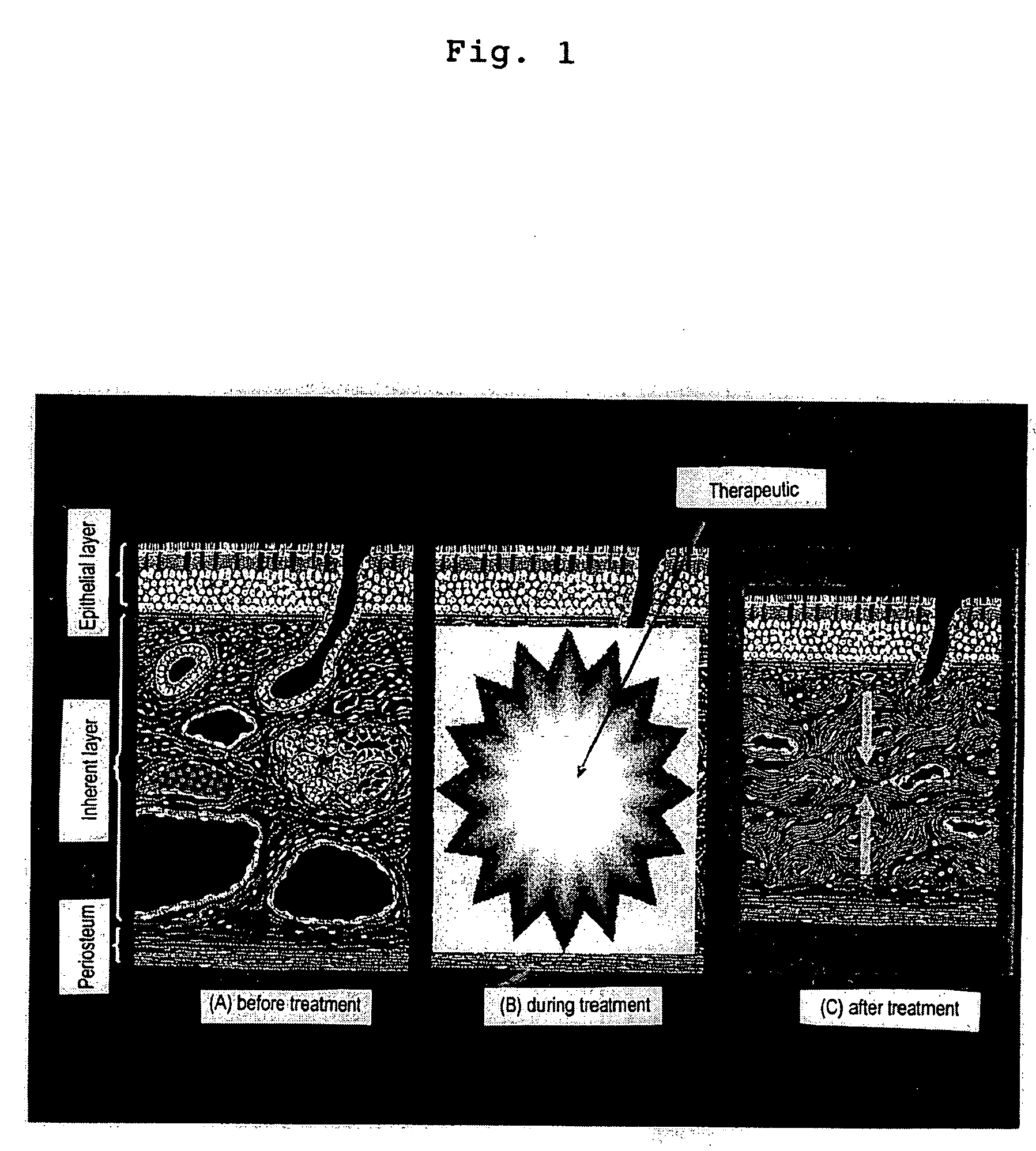
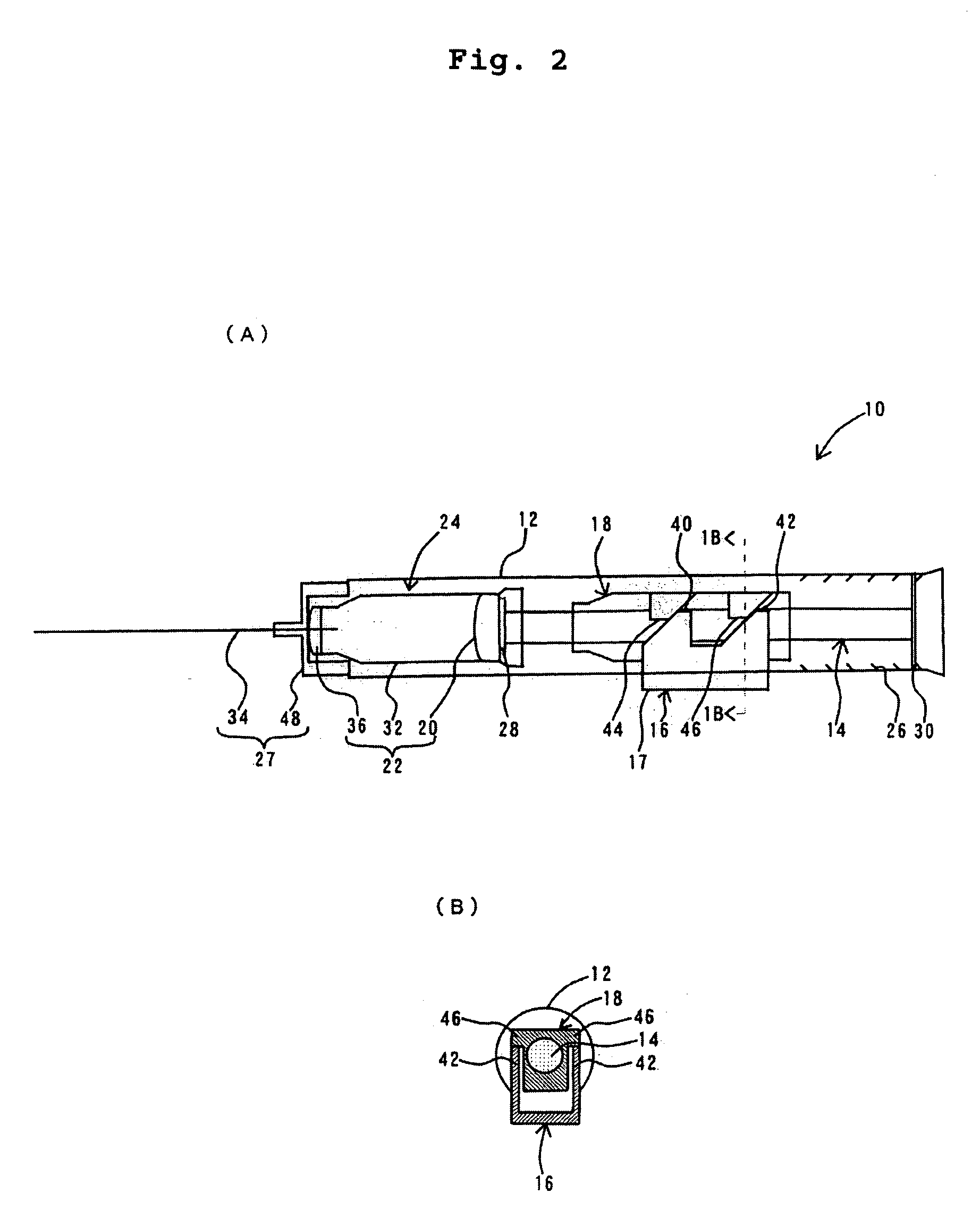
Therapeutic causing contraction of mucosal tissue, method of treating diseases relating to mucosal tissues, injector and therapeutic set
InactiveUS20050187303A1Simple and safe processMinimal invasivenessBiocideHydroxy compound active ingredientsNoseMucosal inflammation
The invention provides for a therapeutic causing contraction of a mucosal tissue whereby various diseases relating to mucosal tissues can be easily, safely and treated with minimal invasiveness, a method of treating various diseases relating to mucosal tissues with the use of the therapeutic causing contraction of a mucosal tissue, and an injector and a therapeutic set usable in the treatment method. The invention also encompasses a therapeutic causing contraction of nasal mucosal tissue containing ethanol as the active ingredient preferably together with a steroid and / or an antihistaminic agent; a method of treating diseases with mucosal inflammation using the above therapeutic causing contraction of a mucosal tissue, and an injector and a therapeutic set usable in the treatment method.
Owner:JAPAN SCI & TECH CORP
Nasal administration preparation containing bupleurum root volatile oil
The present invention belongs to the field of Chinese medicine preparation, and is a kind of nasal administrated preparation containing volatile bupleurum root oil. Proper pharmaceutical supplementary material in certain proportion is used to increase the dissolving degree of volatile bupleurum root oil in water medium while producing no irritation to nasal mucous membrane and toxicity to nasal cilium. The nasal administrated preparation of volatile bupleurum root oil of the present invention has high dissolving degree of volatile bupleurum root oil and low irritation to nasal mucous membrane and toxicity to nasal cilium.
Owner:FUDAN UNIV +1
Black cumin seed oil nose drop and preparation method thereof
ActiveCN103830355AReduce infiltrationConvenient treatmentAntipyreticAnalgesicsSinusitisInflammatory cell infiltration
The invention relates to black cumin seed oil nose drop and a preparation method thereof. The black cumin seed oil nose drop is prepared from black cumin seed oil or from black cumin seed oil, scabrous mosla herb, herba ephedra, radix angelicae, violet magnolia, centipeda minima, schizonepeta and radices sileris in a compounding manner. The black cumin seed oil nose drop disclosed by the invention is used for directly acting on the mucosa of the nasal cavity, becomes effective rapidly, and can relieve inflammatory cell infiltration of the nasal mucosa caused by rhinitis and nasosinusitis and inhibit hyperplasia of connective tissues below the mucosa so as to improve nasal obstruction caused by swelling of the nasal mucosa, so the black cumin seed oil nose drop is suitable for treating various rhinitis and has a better effect than clinical commonly-used biyanling pills, centipeda minima nasal drops and the like.
Owner:徐凌川 +1
Use of cobra venom cardiotoxins in preparation of drug for diminishing inflammation, easing pain and resisting arthritis
The invention discloses a use of cobra venom cardiotoxins in preparation of a drug for diminishing inflammation, easing pain and resisting arthritis. The drug is a single-component preparation or a compound preparation. 30-3000 micrograms per kilogram of the cobra venom cardiotoxins produce good effects of diminishing inflammation, easing pain and resisting arthritis. The cobra venom cardiotoxins have the advantages of small use amount, safety, oral administration, injection administration, oral and nasal mucosa delivery administration and transdermal delivery administration.
Owner:SUZHOU RENBEN PHARMA
Nasal spray of seaweed extract and preparation method of nasal spray
InactiveCN103301065AEasy to prepareEasy to operateOrganic active ingredientsAerosol deliveryBiotechnologyVirus influenza
The invention relates to nasal spray of seaweed extract and a preparation method thereof. The content of active ingredient seaweed polysaccharide of the nasal spray in every millimeter of solution is 1mg-2.5mg; the content of glycerin in every millimeter of solution is 0.1mg-0.3mg; the content of sodium chloride in every millimeter of solution is 4.0mg-5.0mg. The preparation method of the nasal spray comprises the following steps of: sterilizing seaweed polysaccharide; adding injection water and humectants; uniformly stirring; adding the rest of injection water to adjust osmotic pressure; filtering, sterilizing, cooling and filling. The nasal spray disclosed by the invention is convenient to use; the preparation method is simple and easy to operate; the preparation is free of preservative, sterile, stable, capable of keeping nasal mucosa moist and capable of effectively preventing viral flu.
Owner:LIAONING YILING KECHUANG BIOLOGICAL MEDICAL TECH +1
Traditional Chinese medicine liquid preparation for treating rhinitis and preparation method thereof
ActiveCN103933502APromote blood circulationNo side effectsAnthropod material medical ingredientsHydroxy compound active ingredientsSinusitisPharmaceutical Aids
The invention discloses a traditional Chinese medicine liquid preparation for treating rhinitis and a preparation method thereof. The liquid preparation comprises 500 to 800 parts of main liquid preparation, and several parts of auxiliary components and rice vinegar; wherein the main liquid preparation is prepared by decocting main medicinal raw materials such as radix bupleuri, balloonflower root, herba schizonepetae, coptidis rhizoma, and centipede with water, and the auxiliary components comprise a scallion liquid, a garlic liquid, borneol, and ethanol. The liquid preparation is mainly used to treat rhinitis, nasosinusitis, and nasal polyp, and has the advantages of little using amount, user-friendliness, direct act on nasal mucosa, improvement on the blood circulation of nasal mucosa, rapid absorption, prominent curative effect, no side effect, and no drug resistance. The clinical observation results have shown that the cure rate of 2900 clinical treatments on rhinitis, nasosinusitis, and nasal polyp can reach 84% to 85%.
Owner:陈园创 +1
Rhinitis therapeutic instrument
InactiveCN112043968APromote blood circulationTo promote metabolismElectrotherapyLight therapyLight irradiationNasal Cavity Epithelium
The invention discloses a rhinitis therapeutic instrument. The instrument comprises a shell covering a nose, wherein a first belt is connected between the two side edges of the shell, a second belt isconnected between the upper edge of the shell and the middle of the first belt, extending rods extending into a nasal cavity is installed on the lower edge of the rear face of the shell, far infraredlamp beads are installed at the other ends of the extending rods, and massage structures are installed on the rear side face of the shell and located at the Yingxiang acupoint, the Bitong acupoint and nose wings. The instrument has the advantages that the Yingxiang acupoint, the Bitong acupoint and the nose wings are massaged through a massage magnet to stimulate the acupoints to promote blood circulation around the nose, infrared light irradiation is conducted on the interior of the nasal cavity through the far infrared lamp beads, subcutaneous tissue is permeated to expand nasal mucosa capillaries, blood circulation and cell tissue metabolism are promoted, the damaged nasal mucosa is repaired, a damaged nasal mucosa is repaired, nasal cavity inflammation is relieved, so that rhinitis, allergic rhinitis and the like are relieved and treated.
Owner:张宗逵
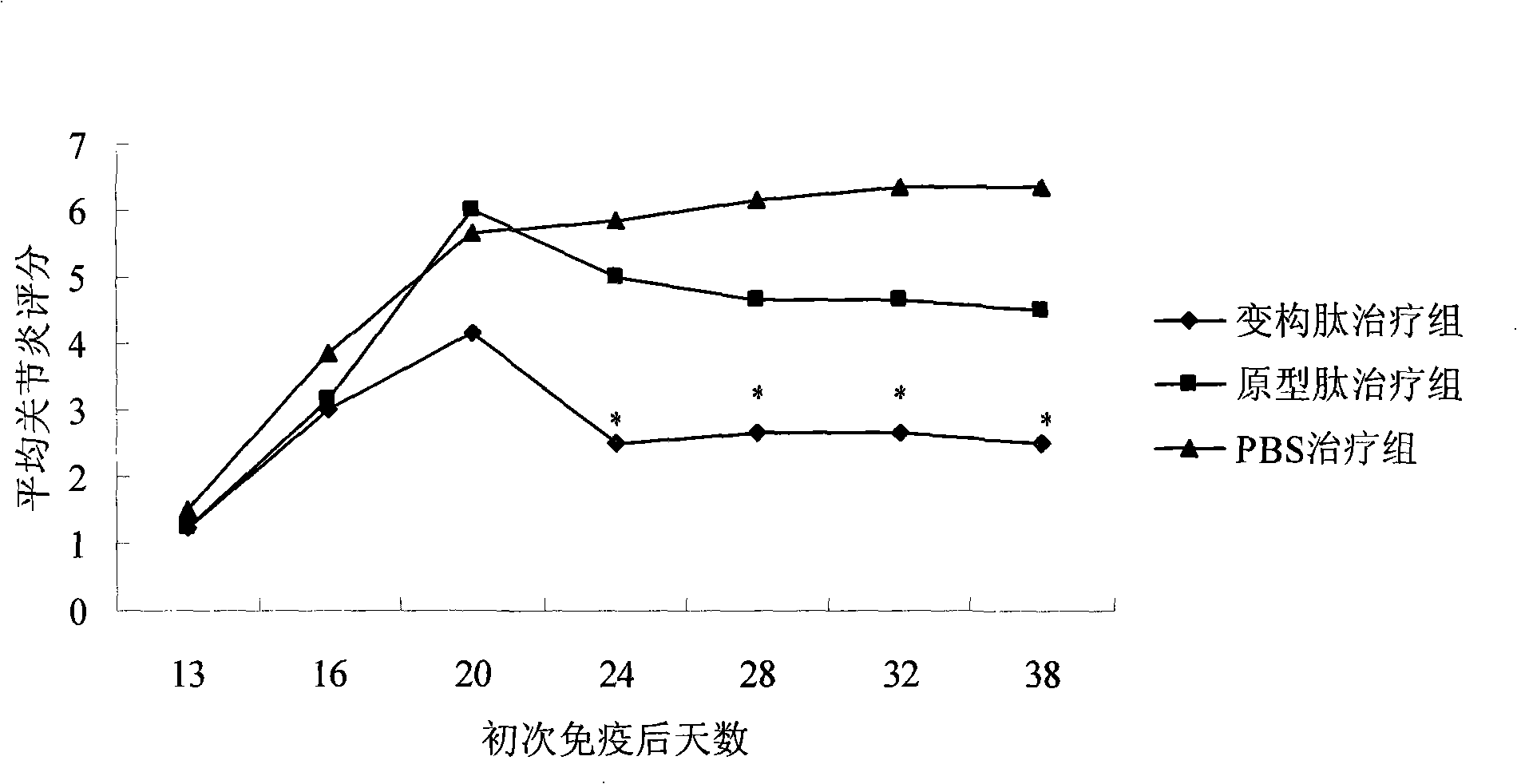
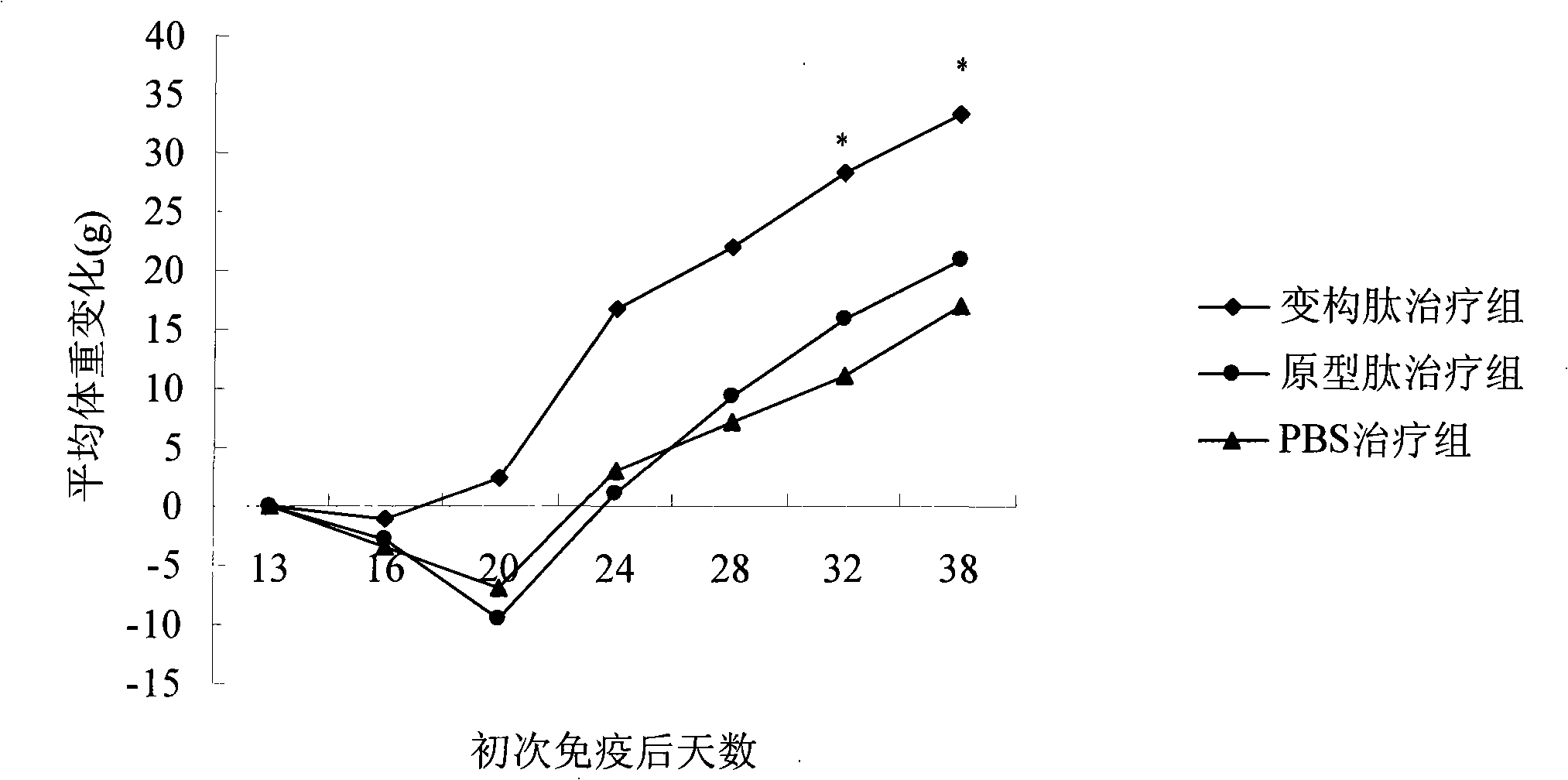
Application of type II collagen allosteric peptide for treating rheumatoid arthritis with nasal mucosa medicine administration
InactiveCN101244257AInhibit joint inflammationProhibition of usePeptide/protein ingredientsAntipyreticType II collagenEarly rheumatoid arthritis
The invention provides a nasal mucosa administration approach using II type collagen altered peptide to cure rheumatoid arthritis, the II type collagen altered peptide nasal mucosa administration specific can inhibit abnormal immune reaction of the rheumatoid arthritis, and control the development of the disease radically aiming at the original tache of disease incidence.
Owner:PEOPLES HOSPITAL PEKING UNIV
Nasal cavity surface preparation
InactiveCN101361857AAct quicklyAerosol deliveryOintment deliveryNasal Cavity EpitheliumPharmaceutical medicine
The invention discloses a preparation for the surface of a nasal cavity, which comprises pimiento and medical ingredients commonly used for pharmacy; by being added with medical materials including fructus xanthii, flos magnolia and liquorice, the preparation can be prepared into a rhinitis curing compound preparation which has a good synergetic effect and acts directly on the nasal mucosa, thus being safe and effective, having fast effects, and be capable of comprehensively controlling and thoroughly improving the symptom of allergic rhinitis.
Owner:GUANGDONG PHARMA UNIV
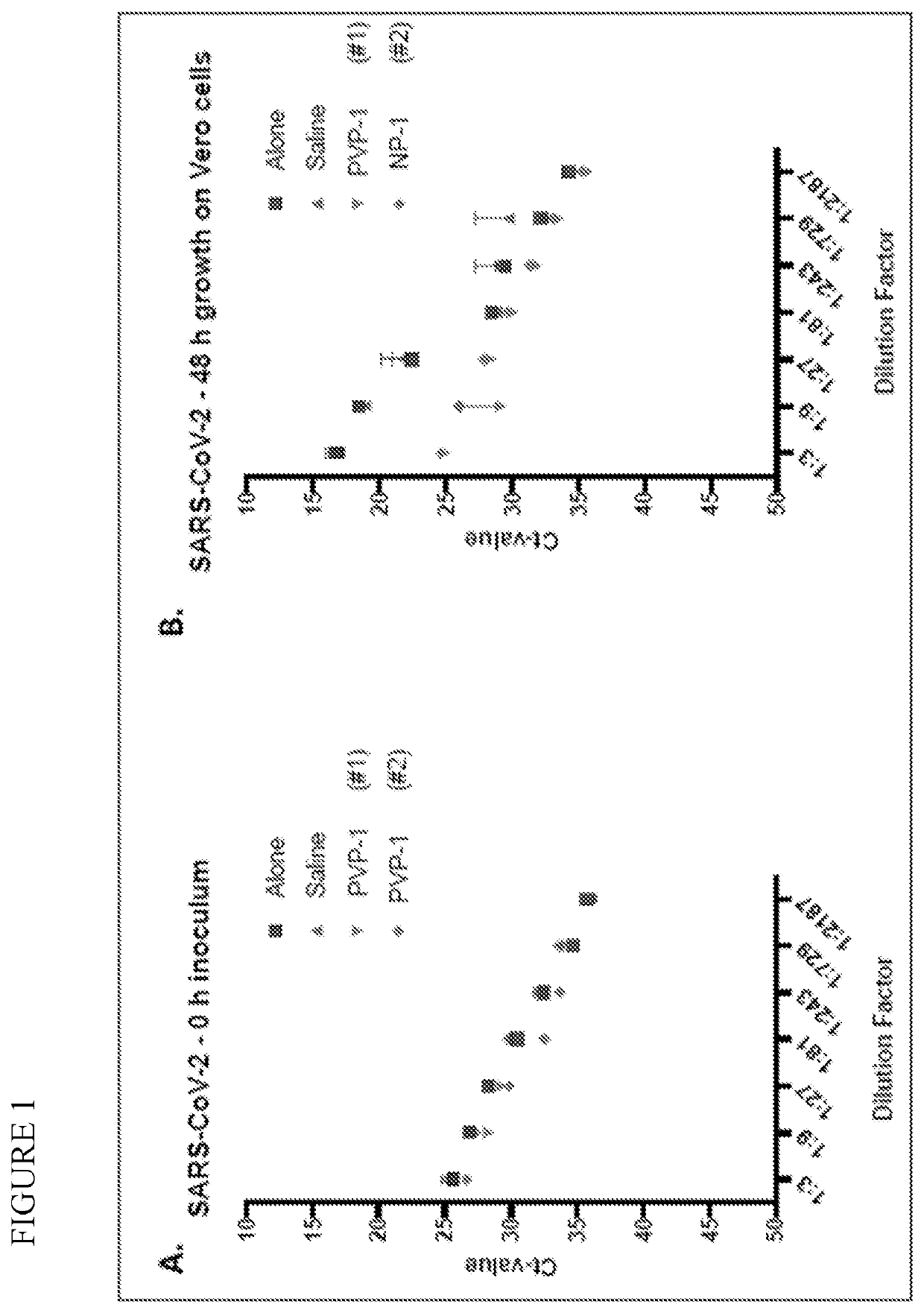
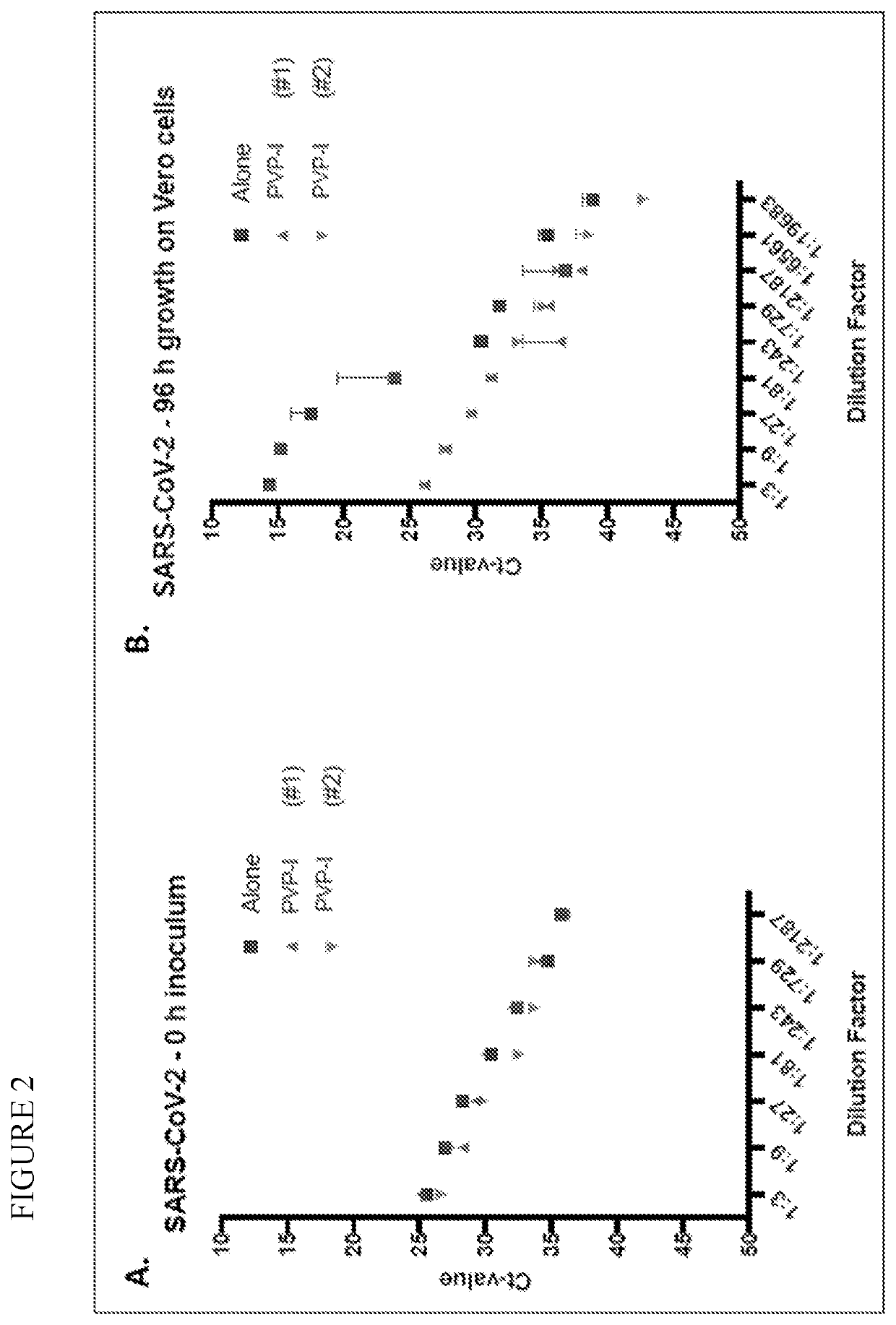
Prevention of infection by highly pathogenic viruses using topical application of povidone-iodine on mucous membranes
ActiveUS20200384016A1Reduce infectionReduce riskAntibacterial agentsDigestive systemHighly pathogenicMicrobial agent
This invention is directed to methods for prevention of infections by highly pathogenic viruses by applying to the nasal mucous membranes topical preparations comprising the broad-spectrum antimicrobial agent povidone-iodine.
Owner:FIREBRICK PHARM LTD
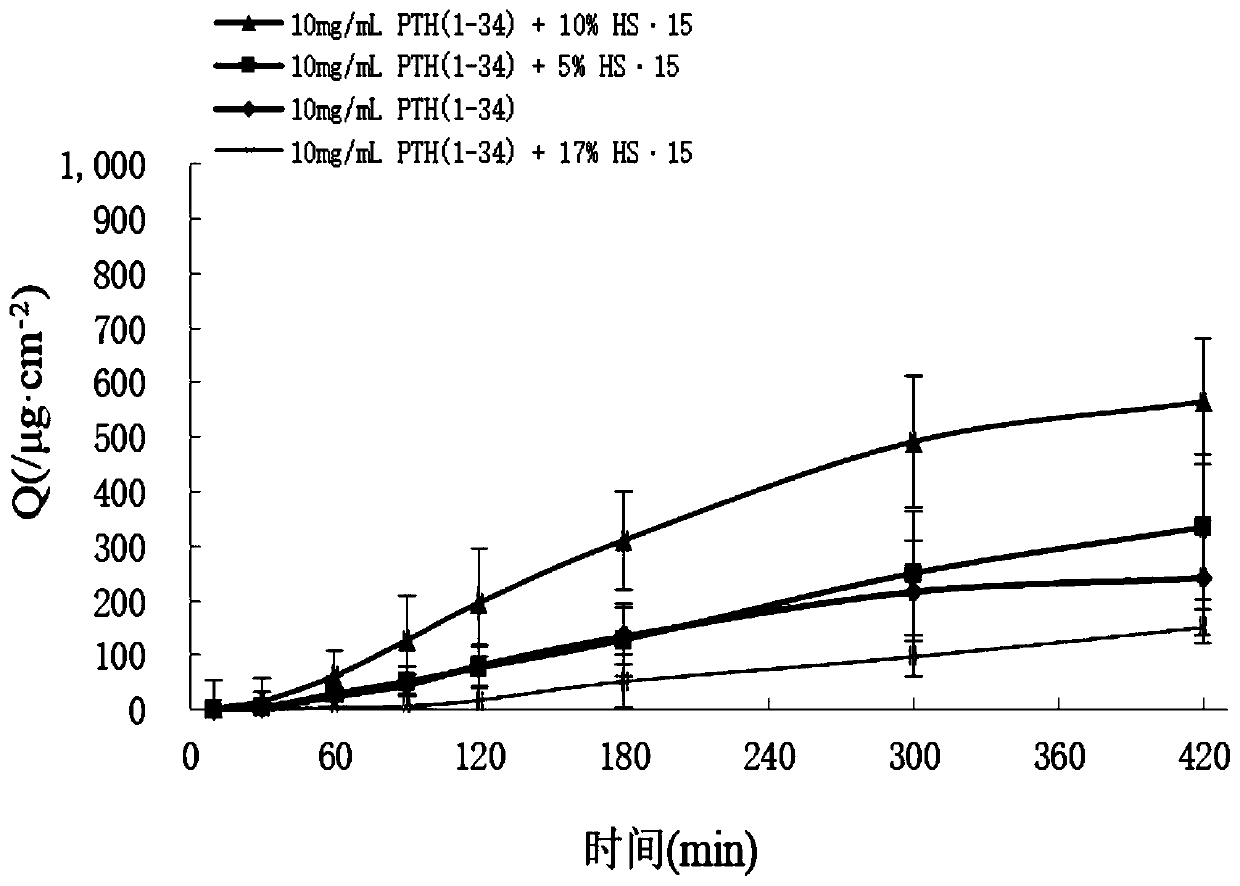
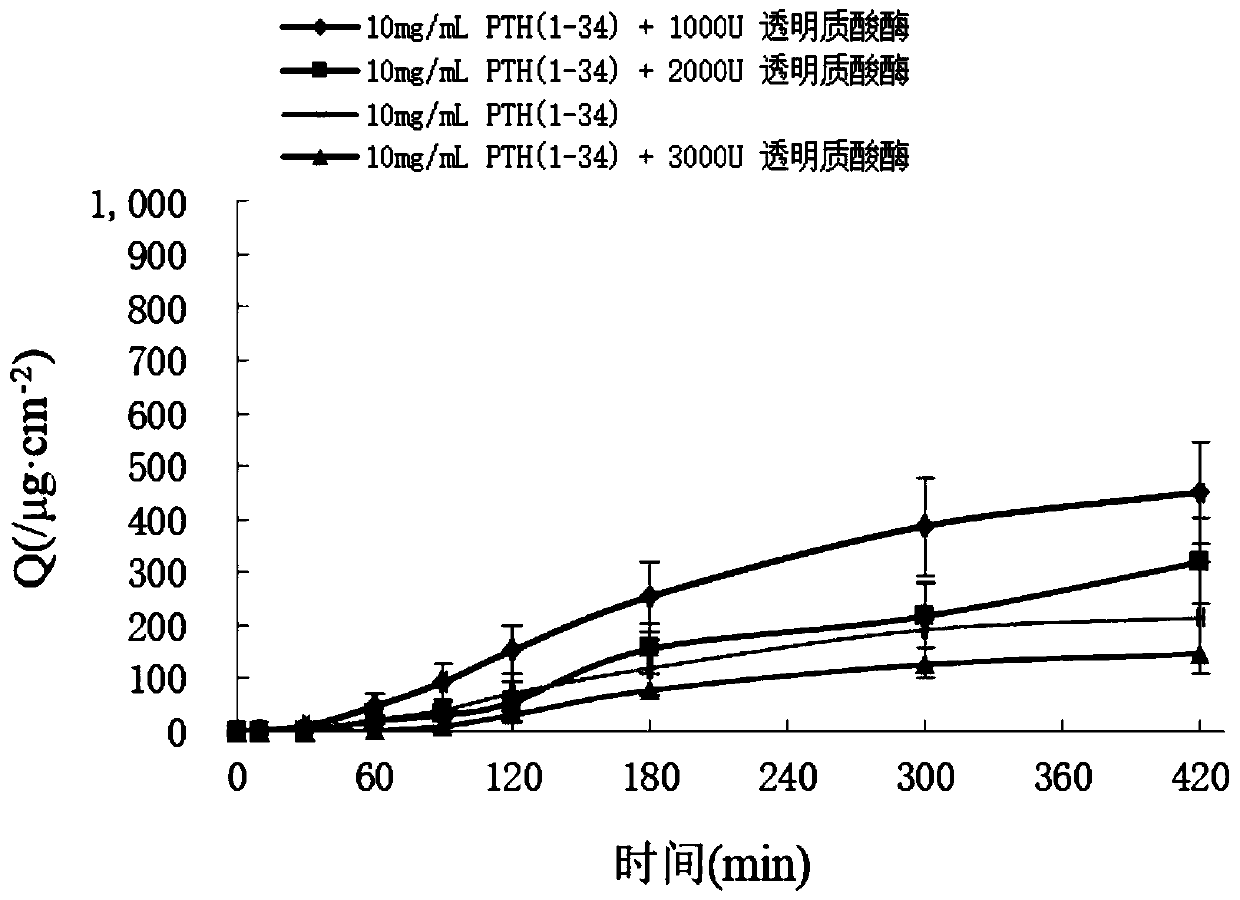
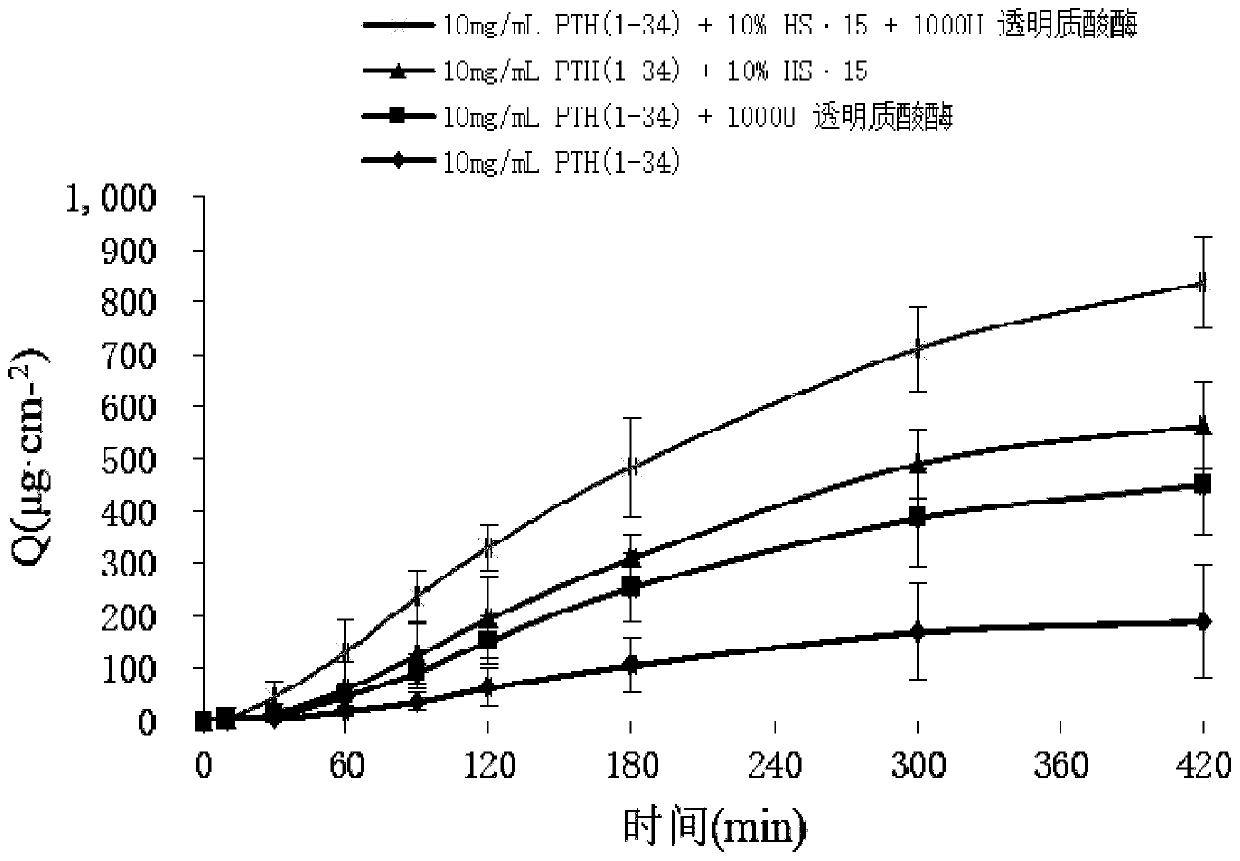
Parathyroid hormone 1-34 nasal spray as well as preparation method and application thereof
ActiveCN110755380APrevention and treatment of osteoporosisImprove compliancePeptide/protein ingredientsAerosol deliveryPeptide drugPatient compliance
The invention relates to a parathyroid hormone 1-34 pharmaceutical composition and a preparation method thereof. Specifically, the invention relates to a parathyroid hormone 1-34 pharmaceutical composition, which contains parathyroid hormone 1-34 as an active ingredient and a pharmaceutically acceptable carrier, and the carrier at least comprises a buffer solution, a penetration enhancer, a stabilizer and a preservative. The parathyroid hormone 1-34 pharmaceutical composition is a parathyroid hormone 1-34 nasal spray or a nasal spray combination. The parathyroid hormone 1-34 nasal spray or thenasal spray combination prepared by the method has the advantages that the first-pass effect is avoided, and the bioavailability is high; the effect taking is fast; the administration is convenient,the patient compliance is strong, the nasal spray is subjected to nasal mucosa absorption in the use process, and can be used for favorably reducing the pain of injection administration of polypeptidedrugs and the like.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Medicine and preparation for treating allergic rhinitis and preparation method of same
InactiveCN108096308AQuick fixEliminate allergy symptomsHydroxy compound active ingredientsMammal material medical ingredientsWestern medicineAllergy
The invention relates to a medicine and a preparation for treating allergic rhinitis and a preparation method of same. The medicine includes Western medicines and traditional Chinese medicines, wherein the Western medicines include, by weight, 38-42 parts of clindamycin and 27-33 parts of lidocaine, and the traditional Chinese medicines include, by weight, 50-100 parts of storax, 15-30 parts of borneol, 10-30 parts of artificial musk, and 50-80 parts of centipeda minima. Through combination of the Western medicines and traditional Chinese medicines, damaged nasal mucosa can be rapidly repairedand allergy symptoms are eliminated. The medicine is convenient to use, has reliable and significant curative effects, is good in safety, and is suitable for adults and childrens.
Owner:袁影丽
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com