Cefotiam hydrochloride/anhydrous sodium carbonate medicinal composition suspension injection and new use thereof
A technology of cefotiam hydrochloride and anhydrous sodium carbonate, which is applied in the field of medicine, can solve the problems of not being able to store for a long time, rapid decomposition of active ingredients, and low bioavailability, and achieve increased drug safety, low price, and simple operation low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
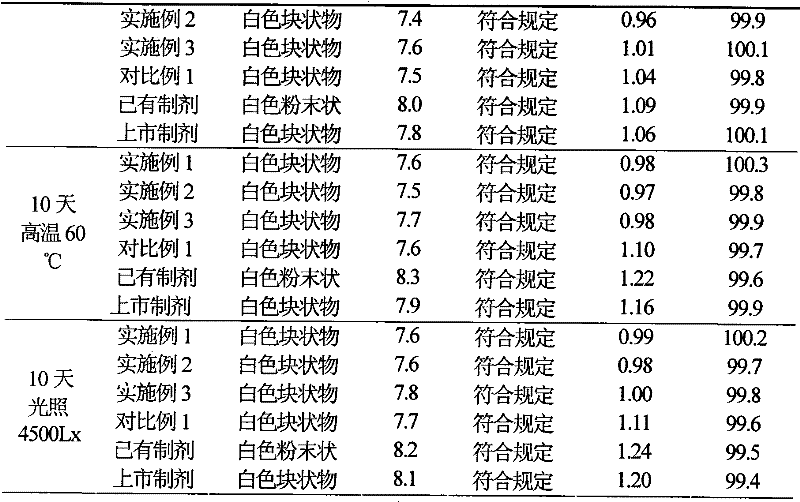
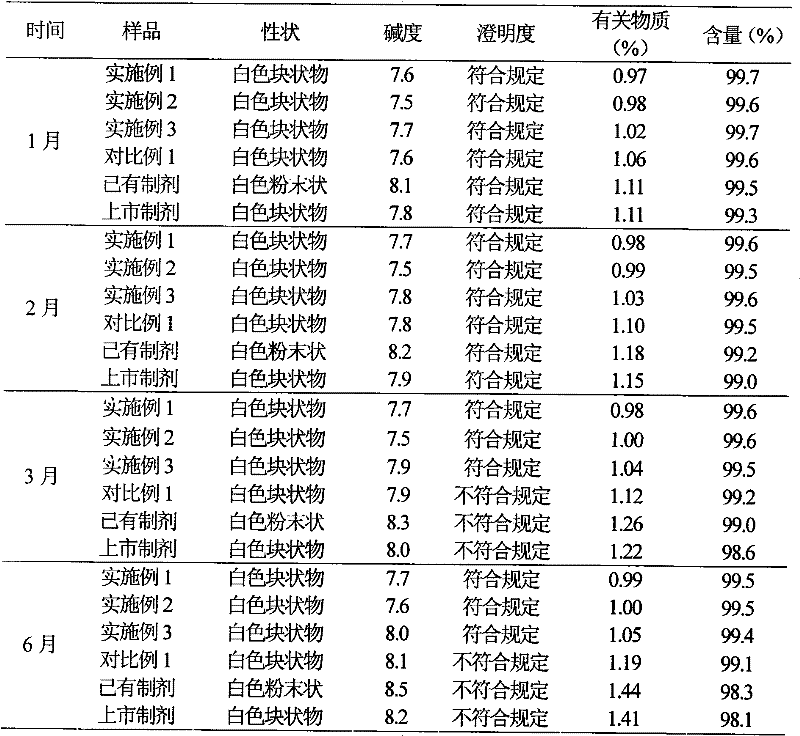
Examples
Embodiment 1
[0036] Example 1 Preparation of Cefotiam Hydrochloride / Anhydrous Sodium Carbonate Pharmaceutical Composition Suspension Injection
[0037] Prescription (specification 0.25g)
[0038] Cefotiam Hydrochloride 25g
[0039] Anhydrous sodium carbonate 5.12g
[0040] Chitosan 50g
[0041] Polyethylene glycol 6000 8g
[0042] Cholesterol 8g
[0043] Mannitol 12g
[0045] Preparation Process:
[0046]A Fully dissolve 25g cefotiam hydrochloride in 500ml water for injection to obtain solution 1, fully dissolve 50g chitosan, 8g cholesterol and 8g polyethylene glycol 6000 in 1000ml ethanol to obtain solution 2, put solution 1 and solution 2 Rotate in a colloid mill for 50 minutes to obtain an emulsion;
[0047] B. Add 12g of mannitol, 3g of sodium sulfite and 5.12g of anhydrous sodium carbonate after steaming the emulsion to remove the organic solvent, then add 500ml of water for injection, stir and dissolve evenly, and pack in vials;
[0048] C is freez...
Embodiment 2
[0049] Example 2 Preparation of Suspension Injection of Cefotiam Hydrochloride / Anhydrous Sodium Carbonate Pharmaceutical Composition
[0050] Prescription (specification 0.5g)
[0051] Cefotiam Hydrochloride 50g
[0052] Anhydrous sodium carbonate 10.24g
[0053] Gelatin 170g
[0054] Povidone K15 8g
[0055] Cholesterol 4g
[0056] Polysorbate 80 4g
[0057] Sodium chloride 10g
[0058] Vitamin E 5g
[0059] Preparation Process:
[0060] A 50g cefotiam hydrochloride was fully dissolved in 600ml water for injection to obtain solution 1, 170g gelatin, 8g povidone K15, 4g cholesterol and 4g polysorbate 80 were fully dissolved in 1500ml isopropanol to obtain solution 2, the solution 1 and solution 2 were placed in a colloid mill and rotated for 50 minutes to obtain an emulsion;
[0061] B. Add 10g of sodium chloride, 5g of vitamin E and 10.24g of anhydrous sodium carbonate after steaming the emulsion to remove the organic solvent, then add 300ml of water for injection, s...
Embodiment 3
[0063] Example 3 Preparation of Suspension Injection of Cefotiam Hydrochloride / Anhydrous Sodium Carbonate Pharmaceutical Composition
[0064] Prescription (specification 0.25g)
[0065] Cefotiam Hydrochloride 25g
[0066] Anhydrous sodium carbonate 5.12g
[0067] Polyamino acid 63g
[0068] Povidone K15 3g
[0069] Polysorbate 80 8g
[0070] Lactose 3g
[0071] Sodium sulfite 1g
[0072] Preparation Process:
[0073] A fully dissolve 25g cefotiam hydrochloride in 450ml water for injection to obtain solution 1, fully dissolve 63g polyamino acid, 3g povidone K15 and 8g polysorbate 80 in 500ml water for injection to obtain solution 2, mix solution 1 and solution 2 Place in a colloid mill and rotate for 45 minutes to obtain an emulsion;
[0074] B. Add 3g of lactose, 1g of sodium sulfite and 5.12g of anhydrous sodium carbonate after steaming the emulsion to remove the organic solvent, then add 550ml of water for injection, stir and dissolve evenly, and then pack in vials; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com