Dissolving ultrafiltration-spray drying-molecule dispersion coating-hydration palletizing-freeze drying method for preparing liposome combination medicine
A molecular dispersion and freeze-drying technology, which is applied in the field of large-scale industrial production of liposome combination drug preparation, can solve the problems of increasing leakage rate, batch fluctuation, energy consumption and time-consuming, etc. The effect of saving workshop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
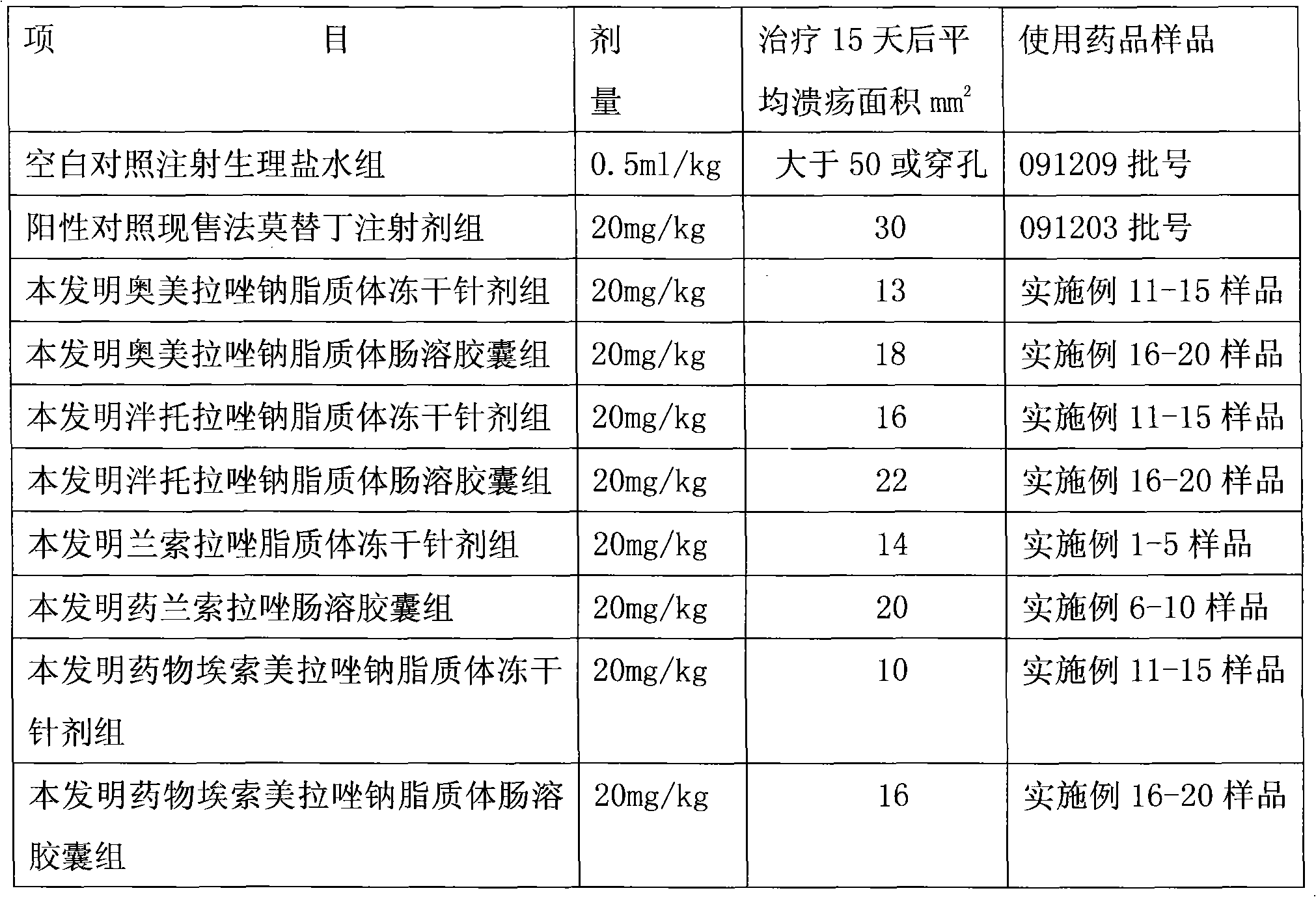
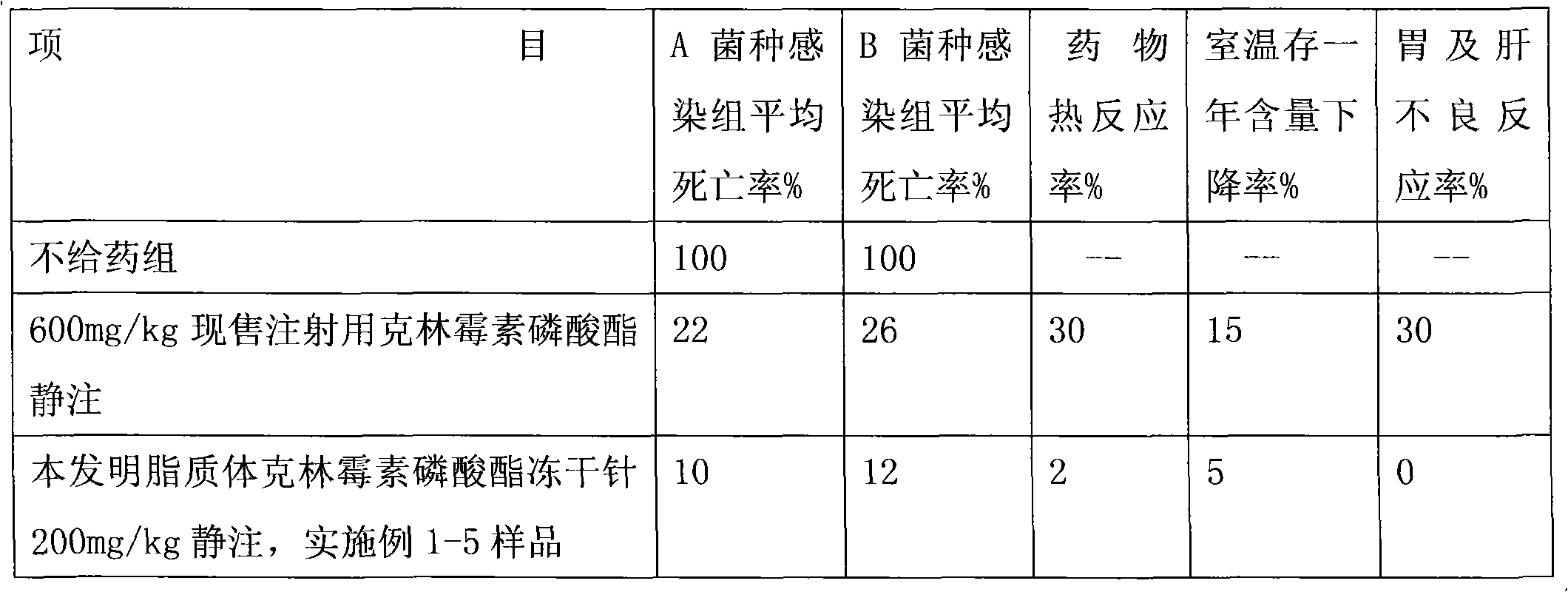
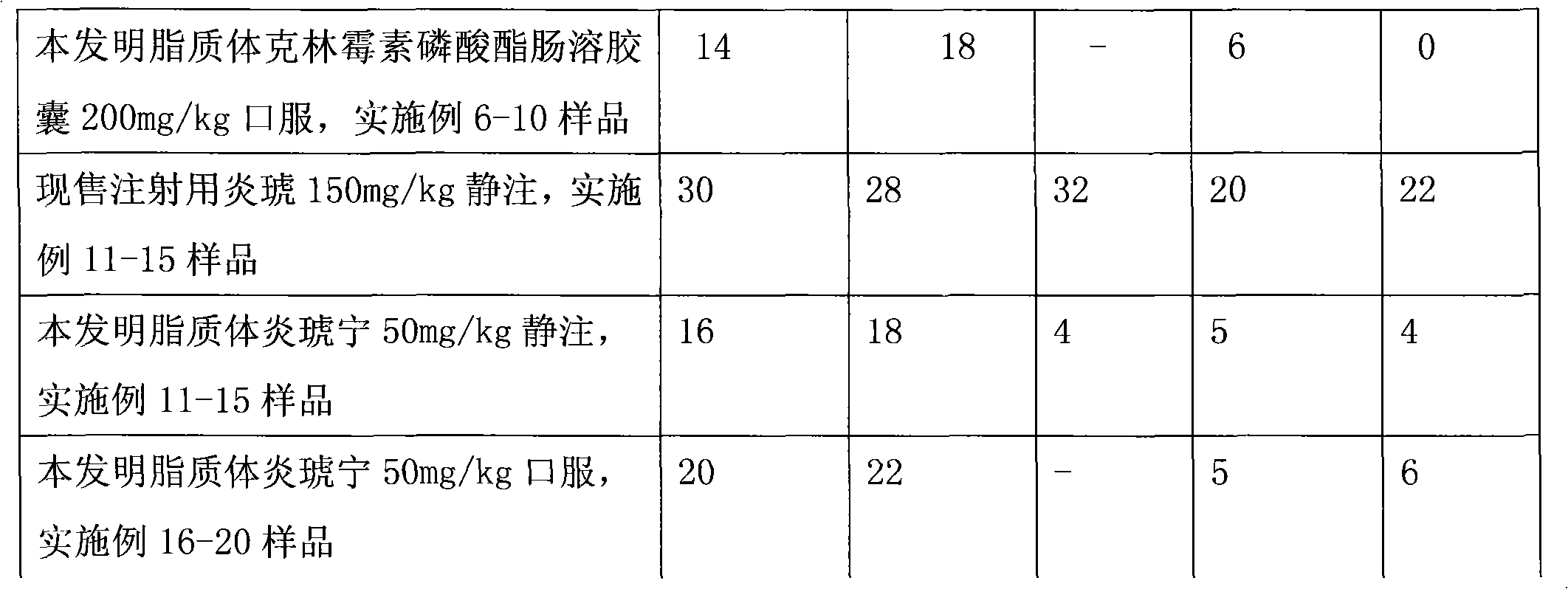
Image
Examples
Embodiment 1
[0056] The raw material drug is a liposome combination drug with strong fat solubility. The molar ratio of each raw material component of the standard is as follows:
[0057] 1. The raw materials are alprostadil and indapamide
[0058] Mole number 1:30 composition 0.05
[0059] 2. Phospholipid raw materials are soybean lecithin and polyene phosphatidylcholine
[0060] Mole ratio 1:0.5 composition 0.50
[0061] 3. The antioxidant is reduced glutathione 0.01
[0062] 4. The molecular diluent of phospholipid membrane is dimercaprol 1.00
[0063] 5. The dispersant and excipient of liposome drug-carrying body is xylitol 2.00
[0064] 6. Surfactant is sodium dehydrocholate 0.01
[0065] 7. Ethanol 80% (v / v) (when dry, evaporate to the fullest) appropriate amount
[0066] 8. Phosphate buffer solution for injection 0.05M pH value 5.0-8.0 appropriate amount
[0067] 9. Equal volumes of water for injection (when dry, evaporate to the limit) and phosphate buffer for injection
[00...
Embodiment 2
[0078] The raw material drug is a liposome combination drug with strong fat solubility. The molar ratio of each raw material component of the standard is as follows:
[0079] 1. The raw materials are alprostadil and indapamide
[0080] Mole number 1:30 composition 0.20
[0081] 2. Phospholipid raw materials are soybean lecithin and polyene phosphatidylcholine
[0082] 5:0.5 molar composition 4.00
[0083] 3. The antioxidant is reduced glutathione 0.06
[0084] 4. The molecular diluent of phospholipid membrane is dimercaprol 6.00
[0085] 5. The dispersant and excipient of liposome drug-carrying body is xylitol 8.00
[0086] 6. Surfactant is sodium dehydrocholate 0.05
[0087] 7. Ethanol 80% (v / v) (when dry, evaporate to the fullest) appropriate amount
[0088] 8. Phosphate buffer solution for injection 0.05M pH value 5.0-8.0 appropriate amount
[0089] 9. Equal volumes of water for injection (when dry, evaporate to the limit) and phosphate buffer for injection
[0090]...
Embodiment 3
[0092] The raw material drug is a liposome combination drug with strong fat solubility. The molar ratio of each raw material component of the standard is as follows:
[0093] 1. The raw materials are alprostadil and indapamide
[0094] Mole number 1:30 composition 0.05
[0095] 2. Phospholipid raw materials are soybean lecithin and polyene phosphatidylcholine
[0096] Mole ratio 2:0.5 composition 4.00
[0097] 3. The antioxidant is reduced glutathione 0.01
[0098] 4. The molecular diluent of phospholipid membrane is dimercaprol 6.00
[0099] 5. The dispersant and excipient of liposome drug-carrying body is xylitol 2.00
[0100] 6. Surfactant is sodium dehydrocholate 0.05
[0101] 7. Ethanol 80% (v / v) (when dry, evaporate to the fullest) appropriate amount
[0102] 8. Phosphate buffer solution for injection 0.05M pH value 5.0-8.0 appropriate amount
[0103] 9. Equal volumes of water for injection (when dry, evaporate to the limit) and phosphate buffer for injection
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com