Escherichia coli protein expression vector using trigger factor as fusion tag and construction method and application thereof
A technology of Escherichia coli and promoter, applied in the field of genetic engineering, can solve the problems of increasing operation steps, difficult to distinguish, difficult to complete the effect of target protein, etc., to achieve the effect of ensuring dose effect and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
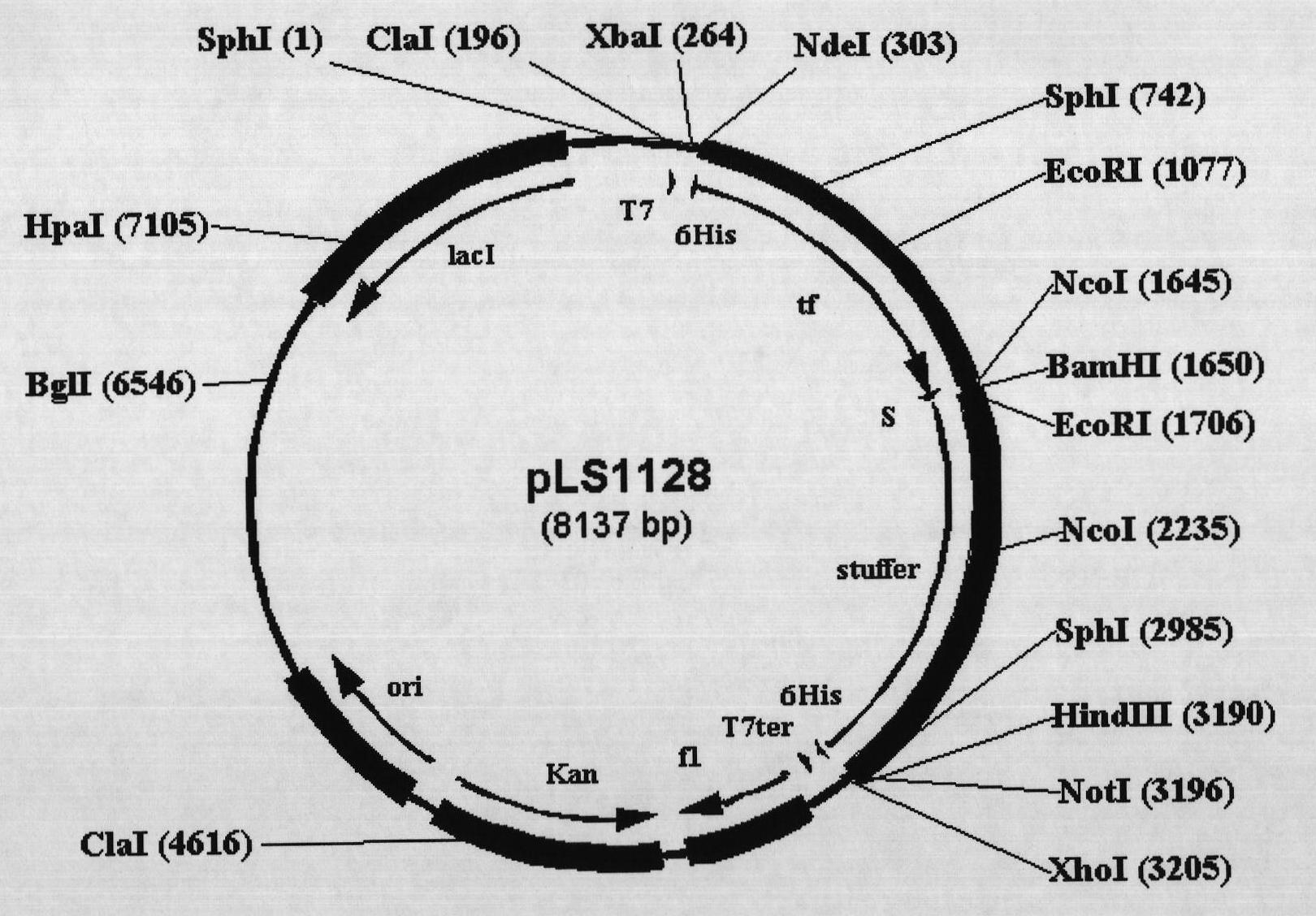
[0060] Example 1. Construction of Escherichia coli protein expression vector pLS1128 with promoter as fusion tag
[0061] 1. Preparation of electrotransformation competent cells of E.coli DH10B, DNA transformation and screening of recombinant clones
[0062] Inoculate a single colony of E.coli DH10B freshly streaked from the plate into 2ml LB liquid medium and shake overnight at 37°C, transfer 1ml to 50ml LB liquid medium, and shake to culture the cells to OD 600 About 0.6. Pour the bacterial solution into a pre-cooled centrifuge tube, place in an ice bath for 10 minutes, centrifuge at 5,000 rpm at 4°C for 5 minutes, and discard the supernatant. The precipitate was washed twice with 10% glycerol, and finally suspended in 200 μl of 10% glycerol, and the prepared electroporation competent cells were filled in 50 μl per tube.
[0063] Add DNA to 50 μl of electroporation-competent cells placed on ice, and mix well by flicking. The mixture was transferred to a pre-cooled 1mm ele...
Embodiment 2
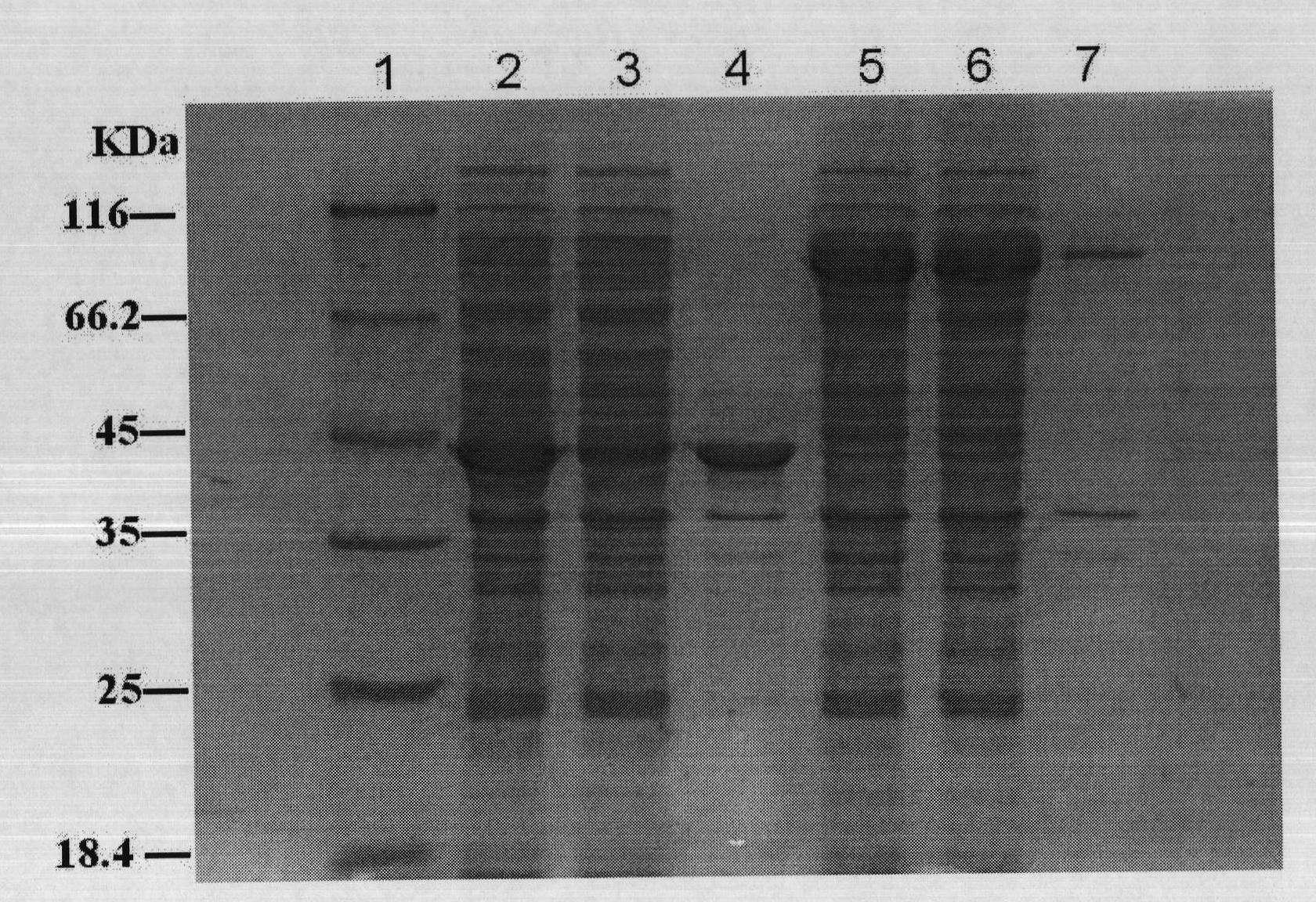
[0089] Fusion expression of embodiment 2.all3695 gene and promoter gene
[0090] 1. Construction of all3695 gene expression strain
[0091] The GenBank accession number of the Anabaena sp. PCC 7120 genome sequence is NC_003272, and the open reading frame sequence of the all3695 gene is the reverse complement sequence of 4461641-4462807. The genomic DNA of Anabaena sp.PCC 7120 was donated by Professor Takakazu Kaneko of Kazusa DNA Research Institute in Japan. Using it as a template and PCA1 and PCA2 as primers, the all3695 gene of 1,167 bp was amplified. The PCR product was double-linked with BamHI-HindIII After digestion and recovery, it was ligated with BamHI-HindIII double-digested pET30a at 16°C, and the ligation product was transformed into competent cells of E.coli DH10B, and recombinant clones were screened on LB plates containing 30 μg / ml kanamycin. After the plasmid was digested and sequenced, it was named pLS1130. The competent cells of E.coli BL21(DE3)plys strain w...
Embodiment 3
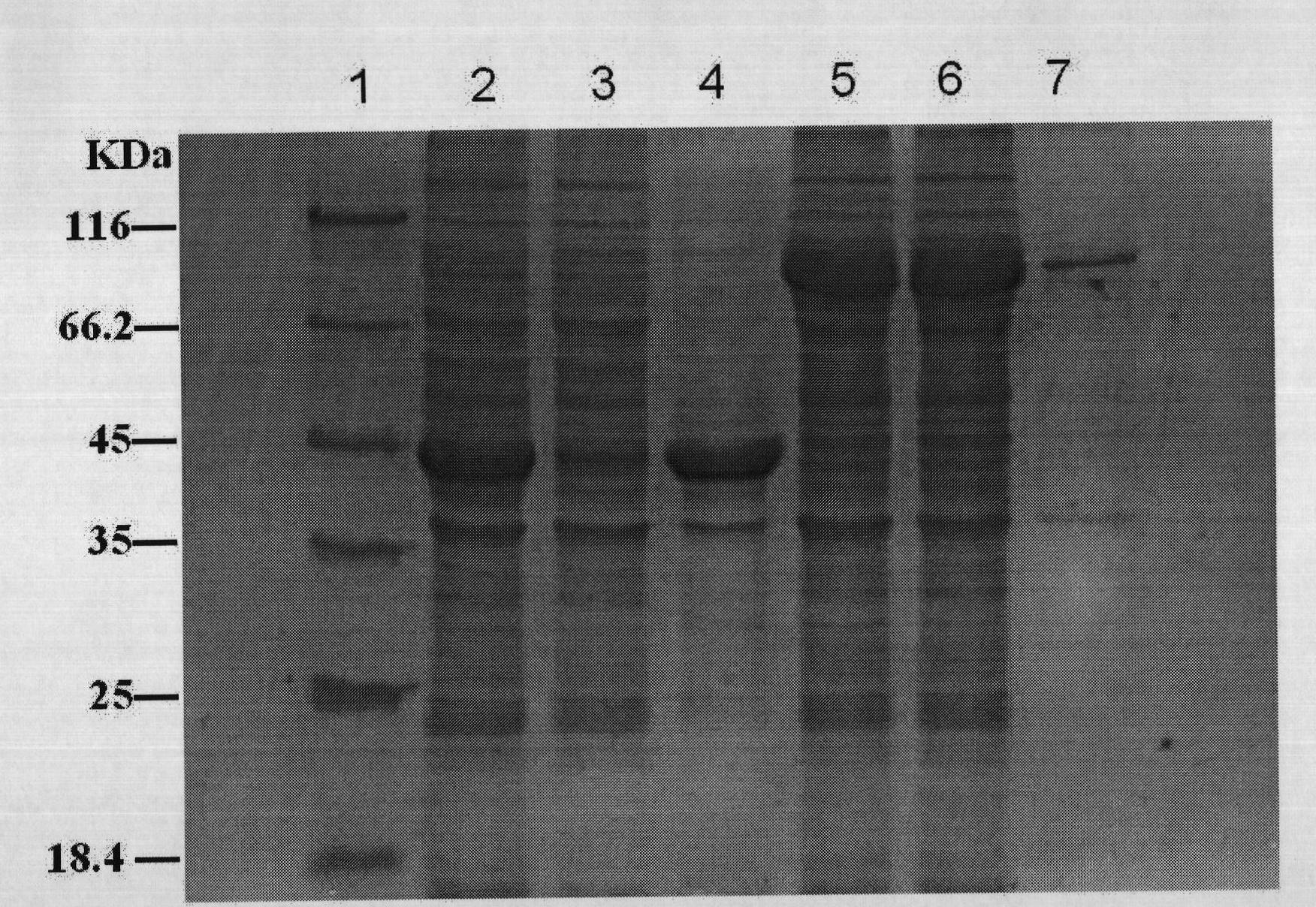
[0103] Embodiment 3. The fusion expression of RB3348 gene and promoter gene
[0104] 1. Construction of RB3348 gene expression strain
[0105] The GenBank accession number of the RB3348 gene is NP_865454, and the cosmid containing the RB3348 gene was donated by Professor Michael Kube of the Max-Planck-Institute in Germany. Using it as a template and RB1 and RB2 as primers, a 1,176bp RB3348 gene was amplified by PCR. After the product was recovered by NcoI-HindIII double enzyme digestion, it was ligated with NcoI-HindIII double enzyme digested pET30a, connected to the competent cells transformed into E.coli DH10B, and the recombinant clone was screened on the LB plate containing 30 μg / ml kanamycin. The plasmid was named pLS1131 after it was verified to be correct by enzyme digestion. The competent cells of E.coli BL21(DE3)plys strain were transformed with pLS1131, and the RB3348 gene expression strain E.coli BL21(DE3)plys / pLS1131 was screened on the LB plate containing 30 μg / m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com