Preparation method of barium titanate hollow nanospheres
A technology of hollow nanometer and barium titanate, applied in the direction of preparation of microspheres, chemical instruments and methods, titanium compounds, etc., can solve the problems of complex reaction procedures, long reaction time, and small amount of products, and achieve simple production process, size and Uniform shape and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
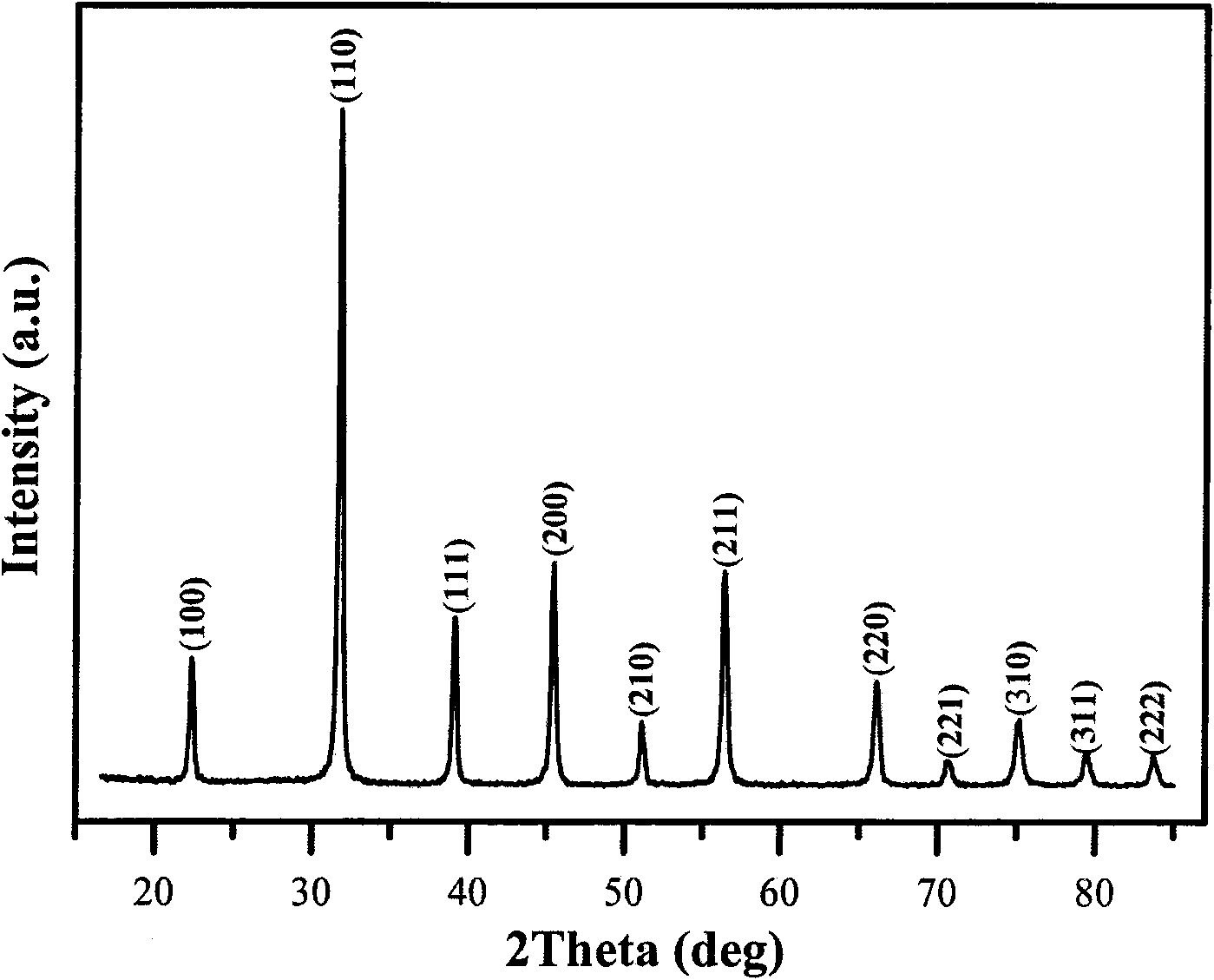
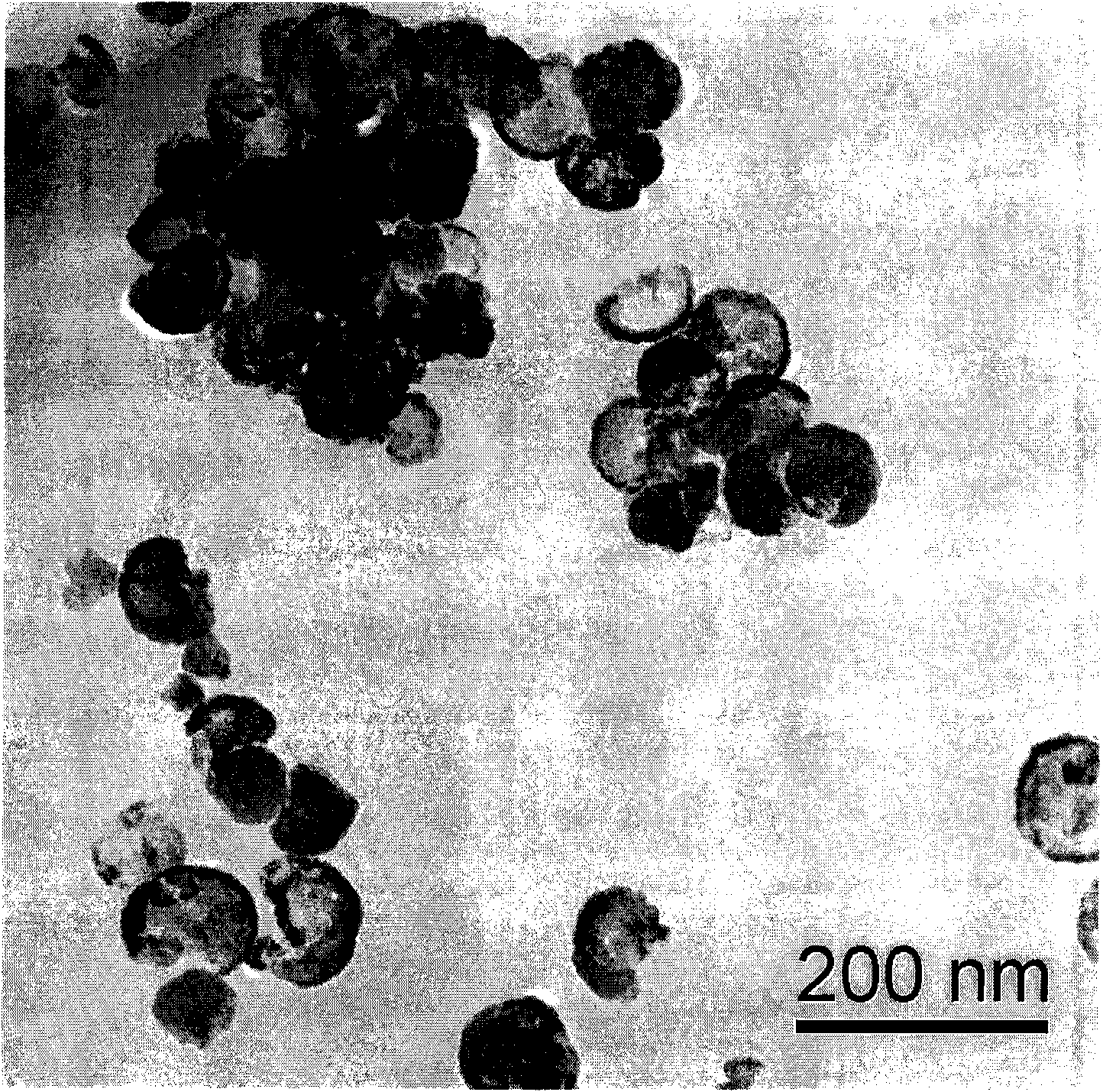
Image
Examples
example 1
[0019] 1) Add 5mL of n-butanol and 45mL of absolute ethanol to a 250mL reaction flask successively, then add 1.701g of tetrabutyl titanate and stir for 10 minutes;
[0020] 2) Weigh 1.314g barium chloride dihydrate and dissolve it in 10mL deionized water;
[0021] 3) Weigh 24.500g of sodium hydroxide and dissolve in 50mL of deionized water;
[0022] 4) under agitation, add step 2) to the reaction flask in step 1) prepared Ba 2+ Ionic solution; after stirring for 30 minutes, add the sodium hydroxide solution obtained in step 3) again; continue stirring for 30 minutes;
[0023] 5) Under stirring, the temperature of the reaction system in step 4) was raised to 70° C. and maintained for 12 hours;
[0024] 6) Stop stirring, allow the product obtained in step 5) to settle naturally, pour out the upper layer solution, and wash the precipitate three times in the order of deionized water, dilute formic acid solution with a volume concentration of 0.5%, and deionized water, and then p...
example 2
[0026] 1) Add 2mL of n-butanol and 40mL of absolute ethanol to a 250mL reaction flask successively, then add 1.698g of tetrabutyl titanate, and stir for 10 minutes;
[0027] 2) Weigh 1.650g of barium hydroxide octahydrate and dissolve it in 10mL of deionized water;
[0028] 3) Weigh 22.000g of sodium hydroxide and dissolve in 50mL of deionized water;
[0029] 4) under agitation, add step 2) to the reaction flask in step 1) prepared Ba 2+ Ionic solution; after stirring for 30 minutes, add the sodium hydroxide solution obtained in step 3) again; continue stirring for 30 minutes;
[0030] 5) Under stirring, the reaction system in step 4) was heated to 100° C., and kept under reflux for 8 hours;
[0031] 6) Stop stirring, allow the product obtained in step 5) to settle naturally, pour out the upper layer solution, and wash the precipitate three times in the order of deionized water, dilute formic acid solution with a volume concentration of 0.9%, and deionized water, and then pl...
example 3
[0033] 1) Add 10mL of n-butanol and 40mL of absolute ethanol to a 250mL reaction flask successively, then add 4.563g of tetraethyl titanate and stir for 10 minutes;
[0034] 2) Weigh 5.292g barium nitrate and dissolve it in 10mL deionized water;
[0035] 3) Weigh 40.000g potassium hydroxide and dissolve in 50mL deionized water;
[0036] 4) under agitation, add step 2) to the reaction flask in step 1) prepared Ba 2+ Ionic solution; after stirring for 30 minutes, add the potassium hydroxide solution obtained in step 3) again; continue to stir for 30 minutes;
[0037] 5) Under stirring, the temperature of the reaction system in step 4) was raised to 60° C. and maintained for 20 hours;
[0038] 6) Stop stirring, allow the product obtained in step 5) to settle naturally, pour out the upper layer solution, and wash the precipitate three times in the order of deionized water, dilute acetic acid solution with a volume concentration of 0.9%, and deionized water, and then place the pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com