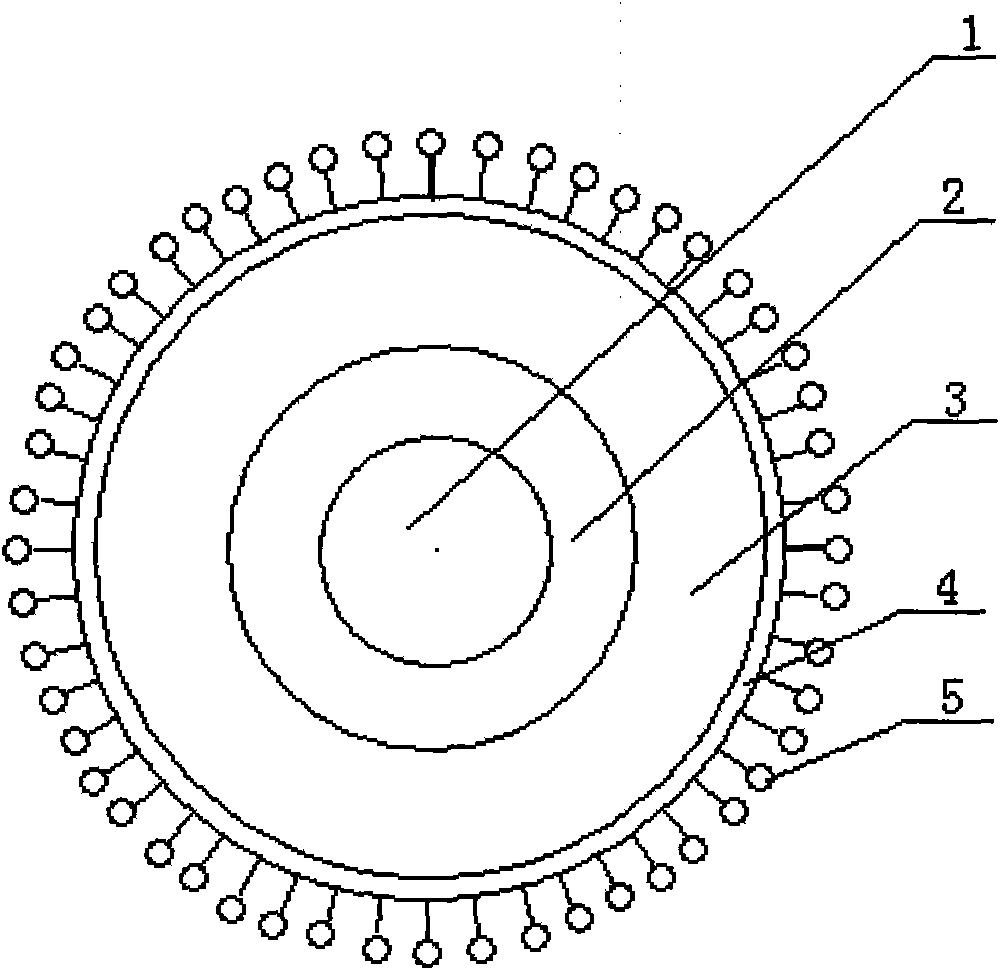
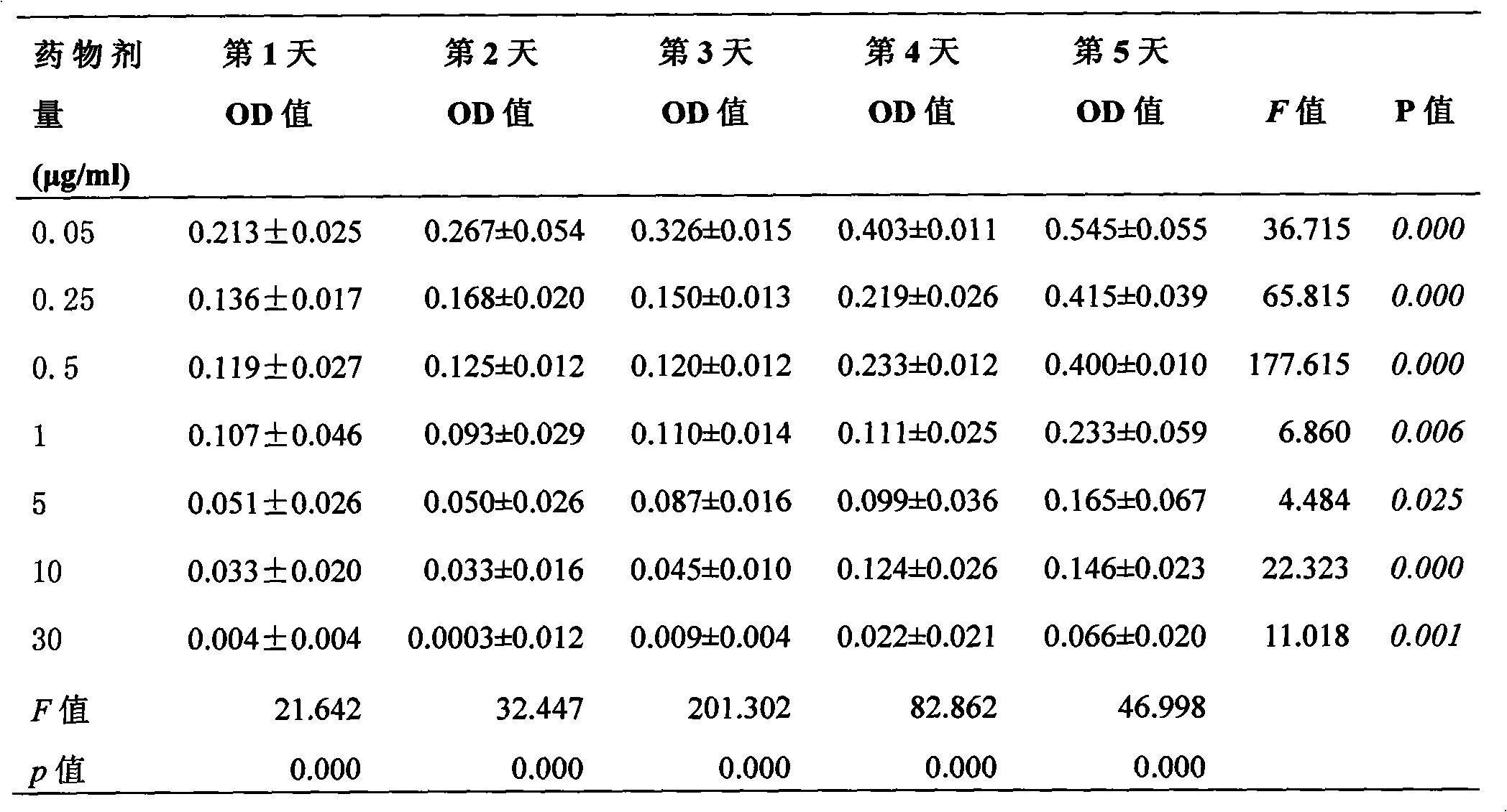
Folic acid-vincristine targeted and slow-released nano-microspheres, prepared method and application thereof
A technology of vincristine and nano-microspheres is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, which can solve the problems of low output, poor stability, high cost, etc. Regular round, smooth surface, uniform size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Folic acid-vincristine targeted sustained-release nano-microspheres are made by the following method:
[0041] (1) 100 mg of polylactic acid-glycolic acid was dissolved in 5 ml of dichloromethane and acetone with a volume ratio of 4:1, and the oil phase was prepared for subsequent use;
[0042] (2) make solvent with deionized water, be mixed with the vincristine aqueous solution that mass concentration is 3%;
[0043] (3) Dissolve 10 mg of polyethylene glycol 4000 and hydroxyethyl cellulose in a mass ratio of 1:3 as an emulsifier in 2.5 ml of the vincristine aqueous solution, and stir in the dark until the emulsifier is completely dissolved to prepare The inner water phase is reserved;
[0044] (4) Add the inner water phase prepared in the step (3) dropwise to the oil phase prepared in the step (1) of 5 ml at 80 watts and ultrasonic dispersion for 2 minutes to form water / oil colostrum solution;
[0045] (5) under the ultrasonic dispersion condition of 120 watts, 3 mi...
Embodiment 2
[0053] Folic acid-vincristine targeted sustained-release nano-microspheres, made by the following method:
[0054] (1) Dissolving 100 mg of polylactic acid-glycolic acid in 4 ml of dichloromethane and acetone with a volume ratio of 5:1, made an oil phase for subsequent use;
[0055] (2) make solvent with deionized water, be mixed with the vincristine aqueous solution that mass concentration is 2%;
[0056] (3) Dissolve 10 mg of polyethylene glycol 4000 and hydroxyethyl cellulose in a mass ratio of 1:4 as an emulsifier in 2.5 ml of the vincristine aqueous solution, and stir in the dark until the emulsifier is completely dissolved to prepare The inner water phase is reserved;
[0057] (4) Add the inner water phase prepared in the step (3) dropwise to the oil phase prepared in the step (1) of 5 ml at 70 watts and ultrasonic dispersion for 3 minutes to form water / oil colostrum solution;
[0058] (5) under the ultrasonic dispersion condition of 110 watts, 4 minutes, the aqueous ...
Embodiment 3
[0062] Folic acid-vincristine targeted sustained-release nano-microspheres, made by the following method:
[0063] (1) 100 mg of polylactic acid-glycolic acid was dissolved in 6 ml of dichloromethane and acetone with a volume ratio of 3:1, and the oil phase was prepared for subsequent use;
[0064] (2) make solvent with deionized water, be mixed with the vincristine aqueous solution that mass concentration is 3%;
[0065] (3) Dissolve 10 mg of polyethylene glycol 4000 and hydroxyethyl cellulose in a mass ratio of 1:2 as an emulsifier in 2.5 ml of the vincristine aqueous solution, and stir in the dark until the emulsifier is completely dissolved to prepare The inner water phase is reserved;
[0066] (4) Add the inner water phase prepared in the step (3) dropwise to the oil phase prepared in the step (1) of 5 ml at 90 watts and ultrasonic dispersion for 1 minute to form water / oil colostrum solution;
[0067] (5) under the ultrasonic dispersion condition of 130 watts, 2 minute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com