Method for preparing 5-hydroxymethyl furfural and 2,5-furandimethanol simultaneously
A technology of furandimethanol and hydroxymethylfuroic acid, which is applied in the direction of organic chemistry, can solve the problems of large safety hazards, expensive catalysts, and high technical requirements for production equipment, and achieve production process safety, low production equipment requirements, and high reaction efficiency. The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] This example provides a method for preparing 5-HMFA and 2,5-FDM from 5-HMF. The base used is KOH. The specific operation is as follows: Weigh 5-HMF (0.5g, 4mmol) into a mortar, add powdered Potassium hydroxide (0.27 g, 4 mmol), triturated at room temperature for 15 min. After the reaction was completed, dilute hydrochloric acid aqueous solution (2ml, 3mol / L) was added to quench the reaction to obtain the product, which was sent to HPLC for detection after adding water to constant volume.
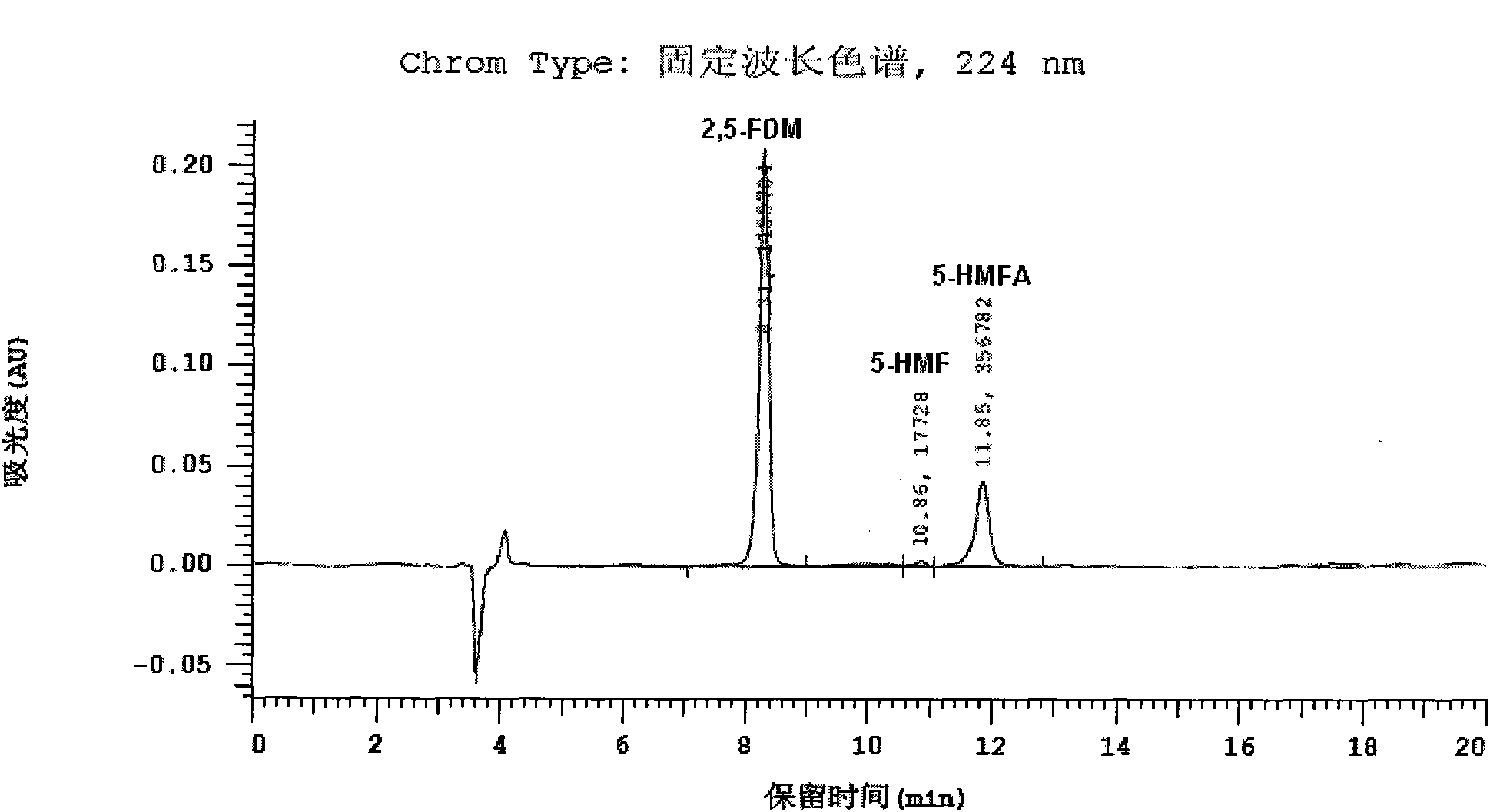
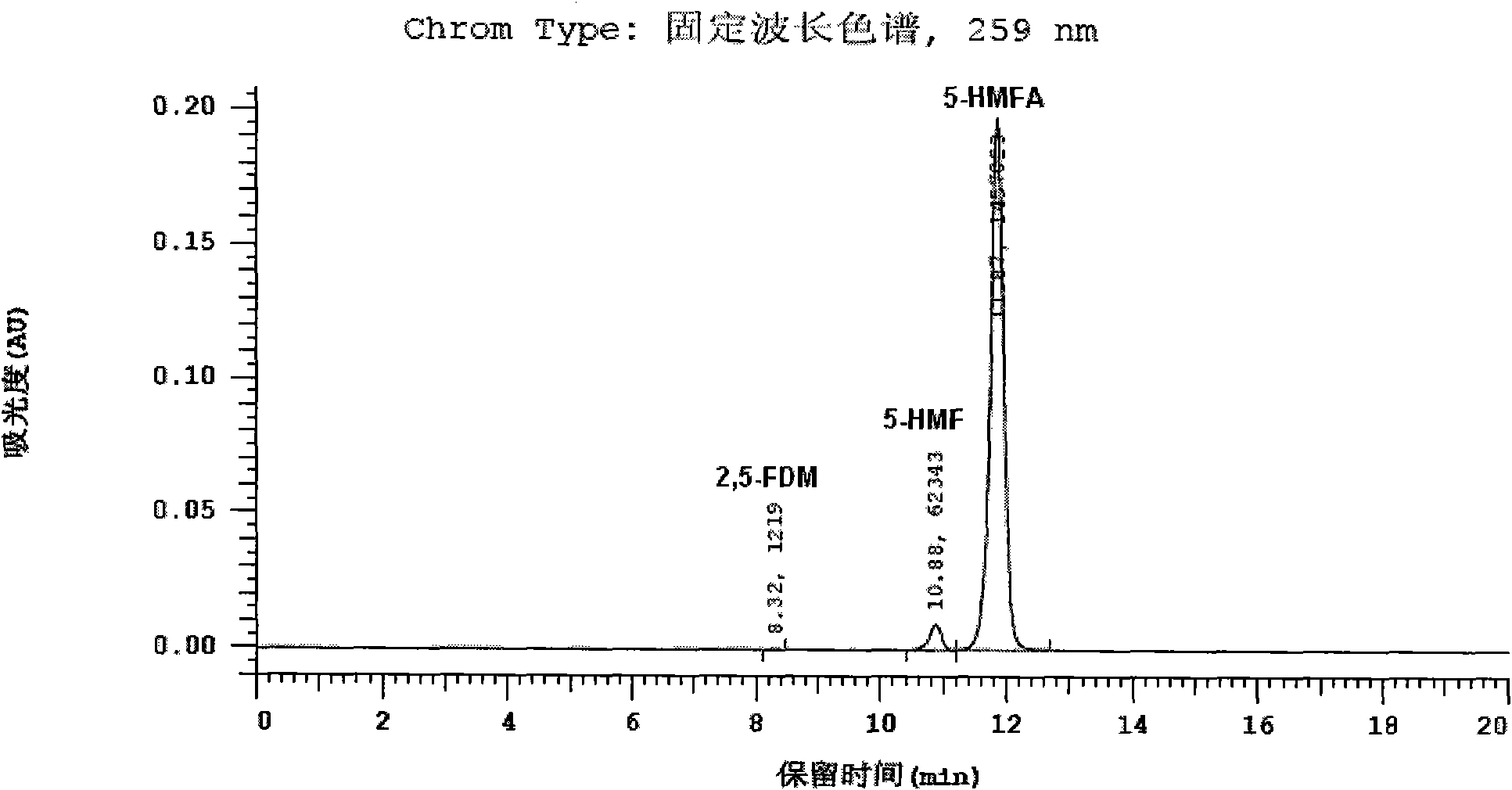
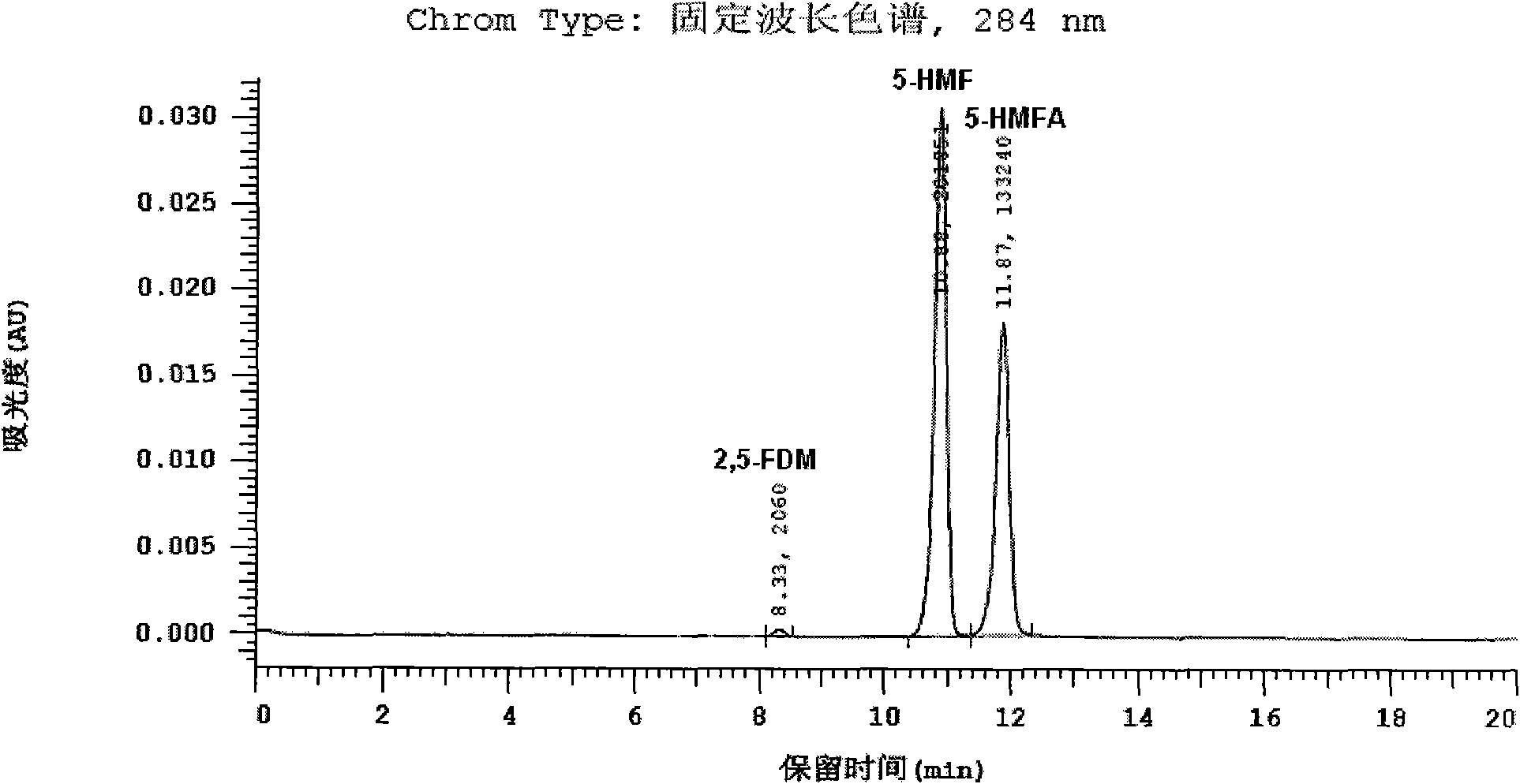
[0056] The specific detection conditions are as follows: liquid phase instrument: Hitachi L-2000 HPLC system, chromatographic column: Cosmosil5C 18 -PAQ column (250×4.6), mobile phase: CH with a volume ratio of 15:85:0.2 3 CN, H 2 A mixture of O and TFA (trifluoroacetic acid), flow rate: 1.0ml / min, column temperature: 30°C, detector: DAD, detection wavelength: 284nm (for detecting 5-HMF), 259nm (for detecting 5-HMF) -HMFA) and 224nm (for detection of 2,5-FDM).
[0057] The resulti...
Embodiment 2
[0059] This example provides a method for preparing 5-HMFA and 2,5-FDM from 5-HMF. The alkali used is NaOH. The specific operation is as follows: Weigh 5-HMF (0.5g, 4mmol) into a mortar, add 0.2ml Water was adjusted to slurry, powdered sodium hydroxide (0.25g, 6mmol) was added, and ground at room temperature for 15min. After the reaction was completed, dilute hydrochloric acid aqueous solution (2ml, 3mol / L) was added to quench the reaction to obtain the product, which was sent to HPLC for detection after adding water to constant volume.
[0060] The specific detection conditions are as follows: liquid phase instrument: Hitachi L-2000 HPLC system, chromatographic column: Cosmosil5C 18 -PAQ column (250×4.6), mobile phase: CH with a volume ratio of 15:85:0.2 3 CN, H 2 A mixture of O and TFA, flow rate: 1.0ml / min, column temperature: 30°C, detector: DAD, detection wavelength: 284nm (for detecting 5-HMF); 259nm (for detecting 5-HMFA) and 224nm (for detection of 2,5-FDM).
[006...
Embodiment 3-10
[0063] According to the same method and conditions as in Example 1, with potassium hydroxide as the base, only the reaction time (grinding time) is replaced according to the different grinding times described in Table 1, and the reaction is completed according to the method provided in Example 1 The system was detected by HPLC, and the conversion rate of 5-HMF, the yield of 5-HMFA and the yield of 2,5-FDM were all listed in Table 1.
[0064] Table 1, the impact of different reaction times on the reaction (alkali: KOH)
[0065]
[0066] As can be seen from Table 1, when potassium hydroxide is used as the base, and its molar amount is the same as that of 5-HMF, after 10 minutes of grinding reaction at room temperature, the conversion rate of raw materials exceeds 90%, and the reaction is basically completed after 30 minutes of grinding reaction.
[0067] The HPLC spectrogram and attached Figure 1-3 There is no substantive difference and will not be described here. From the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com