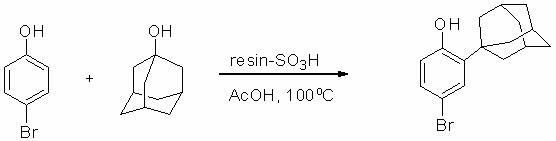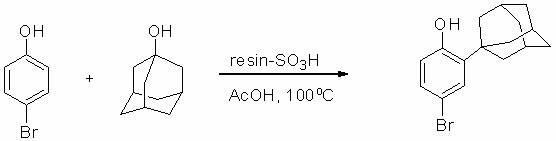Method for preparing 2-(1-adamantyl)-4-bromophenol
A technology of adamantyl and bromophenol, which is applied in chemical instruments and methods, preparation of organic compounds, chemical recovery, etc., can solve the problems of waste water, three wastes in the process, low recovery rate, etc., and achieve high yield and simple process , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Soak 530 grams of D001 sodium-type cation exchange resin (water content 40%) with 500 milliliters of 30% sulfuric acid aqueous solution, filter after 12 hours and wash the resin with deionized water until the eluent is neutral; then vacuum (100mmHg ) to dry the resin, soak the resin with 300 grams of glacial acetic acid for 1 hour, and filter it with suction; then repeat the soaking and suction filtration once, and dry the obtained resin at 100°C for 3 hours in vacuum (10mmHg) to obtain H-type strongly acidic ion exchange resin Catalyst D001 315 grams.
[0021] (2) Add 315 grams of H-type strongly acidic ion exchange resin catalyst D001 obtained in step (1), 173 grams (1.0 mole) of p-bromophenol, 1-adamantane to a 2-liter three-necked flask equipped with a mechanical stirring device at room temperature 157 grams (1.03 moles) of acid and 780 grams of glacial acetic acid, heated to 100 ° C, stirred for 3 hours; after cooling to room temperature, 105 grams of acetic an...
Embodiment 2
[0023] (1) Same as embodiment 1.
[0024] (2) Add 315 grams of the H-type strong acid ion exchange resin catalyst obtained in step (1) to a 2-liter three-necked flask equipped with a mechanical stirring device at room temperature, 173 grams (1.0 mole) of p-bromophenol, 1-adamantanic acid 157 grams (1.03 moles) and 780 milliliters of recovered acetic acid, heated to 100°C, and stirred for 3 hours; after cooling to room temperature, 105 grams of acetic anhydride was added to the reaction mixture, stirred for half an hour, and the ion exchange resin was recovered by filtration, and the mother liquor was recovered by distillation 825 grams of acetic acid; the residue was dissolved in 2 liters of petroleum ether at 60-90°C for thermal recrystallization to obtain 295 grams of product 2-(1-adamantyl)-4-bromophenol with a molar yield of 96%. Product melting point: 147~148 o C, HPLC purity 99.6%. The recovered ion exchange resin, acetic acid and recrystallization mother liquor are al...
Embodiment 3
[0028] (1) Soak 530 grams of 001 (732) sodium type cation exchange resin (water content 40%) with 500 ml of 30% sulfuric acid aqueous solution, filter after 12 hours and wash the resin with deionized water until the eluent is neutral, vacuum (100mmHg) to dry the resin, soak the resin with 300g of glacial acetic acid for 1 hour, and then filter it; then repeat the soaking and suction filtration once, and dry the obtained resin at 100°C for 3 hours under vacuum (10mmHg) to obtain H-type strong acidity 330 grams of ion exchange resin catalyst.
[0029] (2) Add 330 grams of H-type strong acid ion exchange resin catalyst obtained in step (1), 173 grams (1.0 mole) of p-bromophenol, 1-adamantanic acid to a 2-liter three-necked flask equipped with a mechanical stirring device at room temperature 157 grams (1.03 moles) and 800 grams of glacial acetic acid, heated to 100 ° C, stirred and reacted for 5 hours; after cooling to room temperature, 105 grams of acetic anhydride was added to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com