Method for preparing isotope-labeled recombinant C reactive protein
A technology of isotope labeling and reactive protein, which is applied in the preparation of recombinant protein and recombinant C-reactive protein, can solve problems such as unreachable, inaccurate measurement results, incomplete protein digestion, etc., to reduce uncertainty and improve Measurement accuracy, resource saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
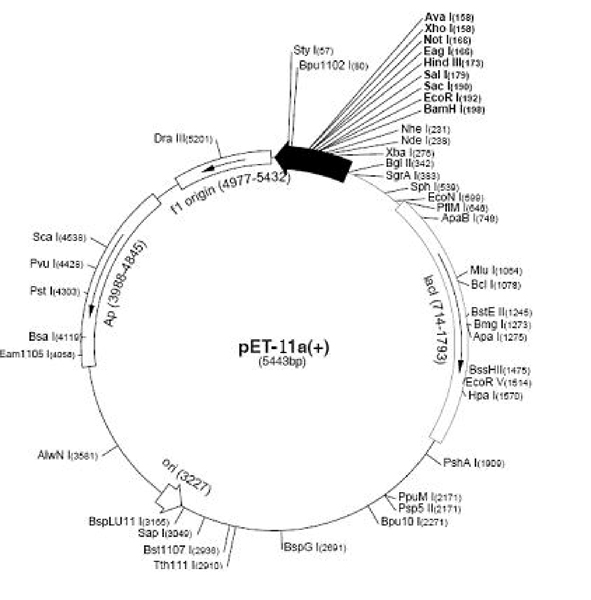
[0024] Example 1 Construction of recombinant vector-pET11a-CRP
[0025] Design primers:
[0026] The upstream primer is R1: AGAAGGAGATATA CATATG CAAACGGACATGTCTC, where the underlined parts in italics are restriction enzymes Nde Ⅰ recognition site;
[0027] The intermediate primer is F1: GTGGTGGTGGTG ACTACCGCG TGGCCACAGCTGCGG, where the underlined part is the nucleotide complementary sequence of 6 histidines, and the blackened part is the recognition site for thrombin digestion; the downstream primer is F2: CTTTGTTAGCAGCC GGATCC TTA GTGGTGGTGGTGGTGGTG ACTA, where the underlined parts in italics are restriction endonucleases Bam The recognition site of HI, in which the nucleotide complementary sequence of 6 histidines in the underlined part, is then amplified by PCR twice to obtain the target gene of C-reactive protein, and its sequence is the core of SEQ ID NO:1 in the sequence table Nucleotide sequence, the coding sequence is in SEQ ID NO: 1, the 7-616 nucl...
Embodiment 2
[0049] Example 2 Small-scale expression of recombinant C-reactive protein
[0050] a. Transform the recombinant vector-pET11a-CRP into the host cell BL21, verify that the host cells containing the recombinant vector are obtained, and then streak the host cells containing the recombinant vector.
[0051] b. Pick a healthy single clone and inoculate it in 5mL LB or TB (Amp: Ampicillin 100μg / mL) at 37°C, 250rpm and culture overnight.
[0052] c. Expand the culture to 50mL LB (Amp100μg / mL) at a ratio of 1:100 (v / v), a total of 4 bottles, shake at 37°C and 250rpm until OD600=0.5-0.6, and add IPTG to two of the bottles until the end Concentration of 1mM was then placed at 20°C and 37°C for shaking culture, and the other two bottles were added with 1% galactose (lacose) for induction and placed at 20°C and 37°C for shaking culture respectively.
[0053] d. After induction at 37°C for 5 hours and overnight at 20°C, take 1-2 mL of bacterial solution, centrifuge at 8000 rpm, 4°C for 5...
Embodiment 3
[0060] Example 3 Massive expression and affinity chromatography purification of recombinant C-reactive protein
[0061] The buffer used in the following test steps is as follows: Lysis buffer: 10mM tris(hydroxymethyl)aminomethane-hydrochloric acid (Tris-Cl) pH8.0, 1mM ethylenediaminetetraacetic acid (EDTA: Ethylene Diamine Tetraacetic Acid ), 0.5% polyethylene glycol octylphenyl ether (triton), 1mM phenylmethylsulfonyl fluoride (PMSF); buffer A: 50mM Tris-ClpH8.0, 500mM NaCl, 8M urea (Urea); buffer B: 50mM Tris-Cl PH8.0, 500mM NaCl, 0.5%triton; Buffer C: 50mM Tris-Cl PH8.0, 150mM NaCl, 400mM imidazole; Dialysis buffer: 50mM Tris-Cl PH8.0, 150mM NaCl, 1mM EDTA, 1mM MDTT, 10% glycerol.
[0062] 1) Pick the healthy single clone obtained in step a of Example 2 above and inoculate in 10mL LB Amp (100μg / mL) at 37°C, 250rpm and culture overnight;
[0063] 2) 1:100 (v / v) expanded culture to 250mL LB (Amp100μg / mL), a total of 2 bottles, 37 ° C, 250rpm shaking culture to OD600 = 0.5 ~...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com