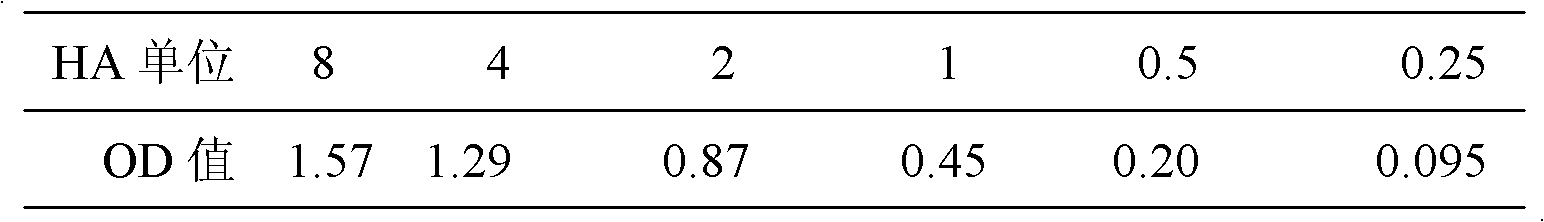H1N1 swine influenza virus-resistant hemagglutinin protein monoclonal antibody, hybridoma cell line and antigen-capture ELISA kit
A hybridoma cell line and hemagglutinin protein technology, applied in the field of immunology, can solve the problems of high requirements for detection equipment and professional knowledge of personnel, potential biological safety hazards, and long time-consuming virus isolation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1, preparation of anti-H1N1 swine influenza virus specific monoclonal antibody
[0015] In the present invention, the monoclonal antibody is prepared using hybridoma technology, specifically as follows:
[0016] a. Animal immunity
[0017] The formaldehyde inactivated H1N1 subtype swine influenza virus was used to immunize Balb / c mice for three times.
[0018] b. Cell Fusion
[0019] Spleen cells from immunized mice were isolated and fused with SP2 / 0 myeloma cell line.
[0020] c. Screening of positive hybridoma cell lines
[0021] Add mouse peritoneal feeder cells to co-culture with fusion cells, and screen for positive strains.
[0022] d. Cloning Screening by Limiting Dilution
[0023] The positive wells were cloned by limiting dilution until a hybridoma cell line capable of stably secreting anti-H1N1 swine influenza virus hemagglutinin protein was obtained.
[0024] The invention obtains a hybridoma cell strain capable of stably secreting and highly...
Embodiment 2
[0027] Example 2, Development of H1N1 Swine Influenza Virus Hemagglutinin Protein Capture ELISA Detection Kit
[0028] 1. Composition of H1N1 Swine Influenza Virus Hemagglutinin Protein Capture ELISA Kit
[0029] The H1N1 swine influenza virus hemagglutinin protein capture ELISA detection kit of the present invention consists of: a solid phase carrier that has been coated with the monoclonal antibody of the present invention, that is, an ELISA plate, and horseradish peroxidase-labeled anti-H1N1 pig Influenza virus hemagglutinin protein polyclonal antibody, enzyme substrate reaction solution, positive and negative controls, washing solution and reaction stop solution.
[0030] 1) ELISA strip coated with anti-H1N1 swine influenza monoclonal antibody
[0031]Dilute the purified monoclonal antibody of the present invention to a concentration of 10 μg / mL with a coating buffer (0.05mol / L carbonate buffer, pH 9.6), and coat a 96-well EIA / RIA high-efficiency binding enzyme plate (Co...
Embodiment 3H1
[0062] Example 3 Specificity and Sensitivity Determination of H1N1 Swine Influenza Virus Hemagglutinin Protein Capture ELISA Detection Kit
[0063] 1) Specificity determination (the following experimental materials were inactivated at 56°C for 30 minutes)
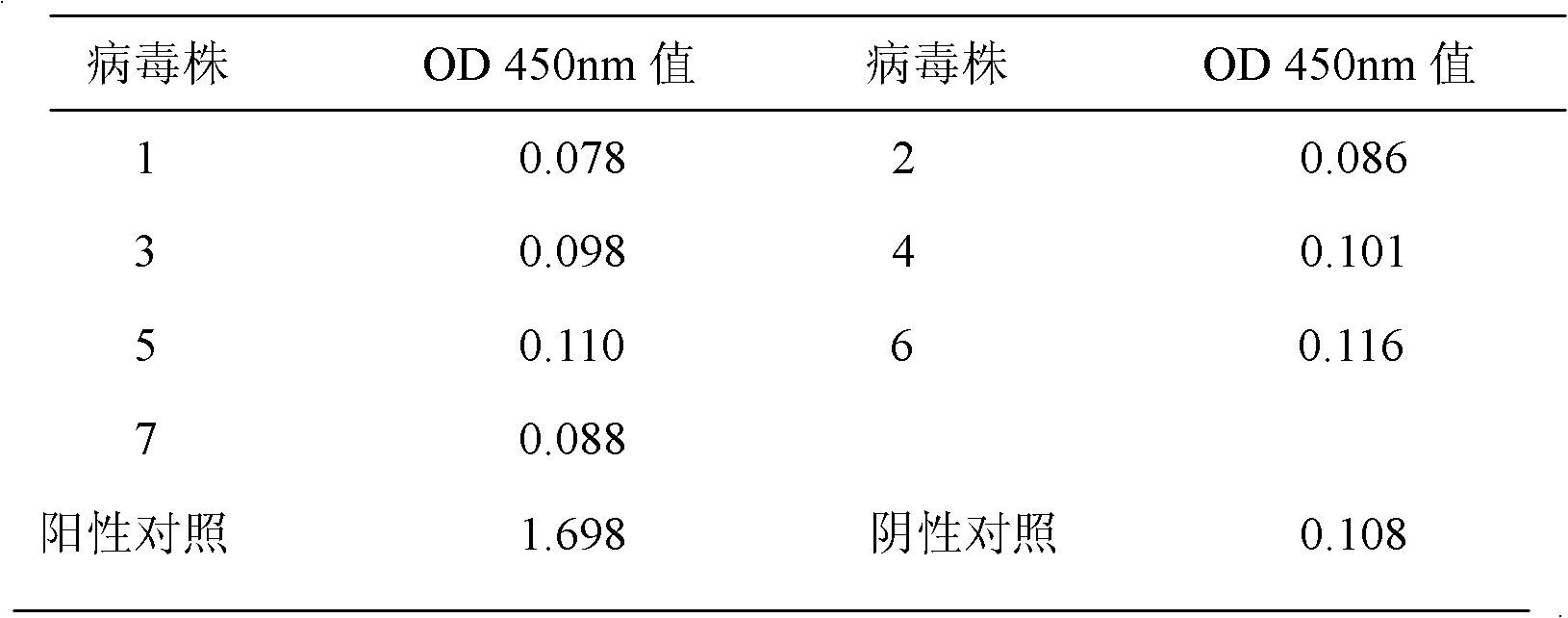
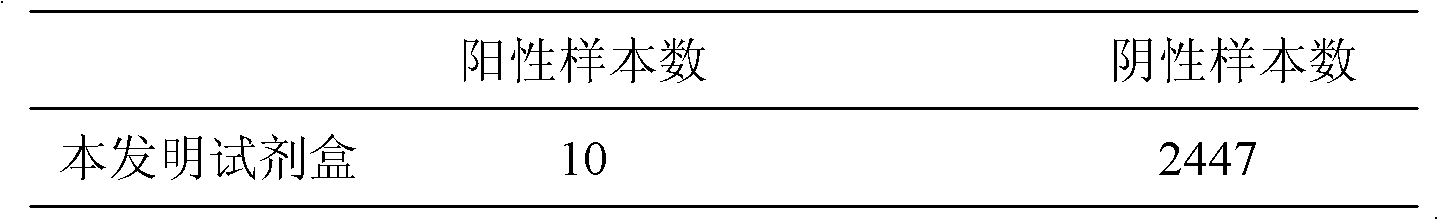
[0064] In order to verify the specificity of the H1N1 swine influenza virus hemagglutinin protein capture ELISA detection kit of the present invention, according to Example 2, 2 strains of Newcastle disease virus samples (No. 1, 2), 2 strains of H3N2 virus samples (No. 3, 4) ), 1 strain of H5N1 virus sample (No. 5), 1 strain of H7N1 virus sample (No. 6) and 1 strain of H9N2 virus sample (No. 7) were tested. The results show that the detection OD value of the kit of the present invention for the above-mentioned protein samples is between 0.078-0.116 (Table 1), showing good specificity.
[0065] Table 1 Specific detection of the kit
[0066]
[0067] 2) Sensitivity determination
[0068] The H1N1 virus allantoic fluid w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com