Bergenin osmotic pump tablet and preparation method thereof
A technology of petracenin and osmotic pump tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the cumbersome preparation process of double-layer osmotic pump preparations and the fluctuation of blood drug concentration Larger, more times of medication, etc., to achieve the effect of improving bioavailability, reducing the number of medications, and reducing the number of medications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1 Preparation of petracenin osmotic pump tablet of the present invention
[0053] 1. Preparation process of petracenin osmotic pump tablets
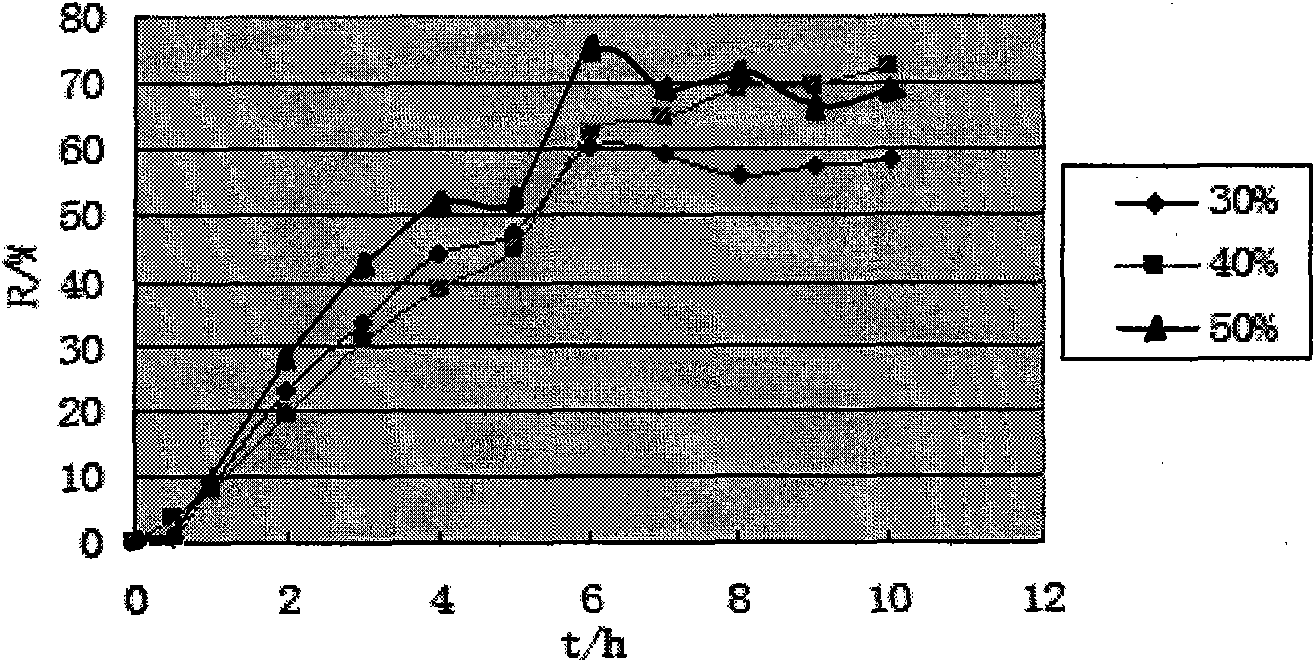
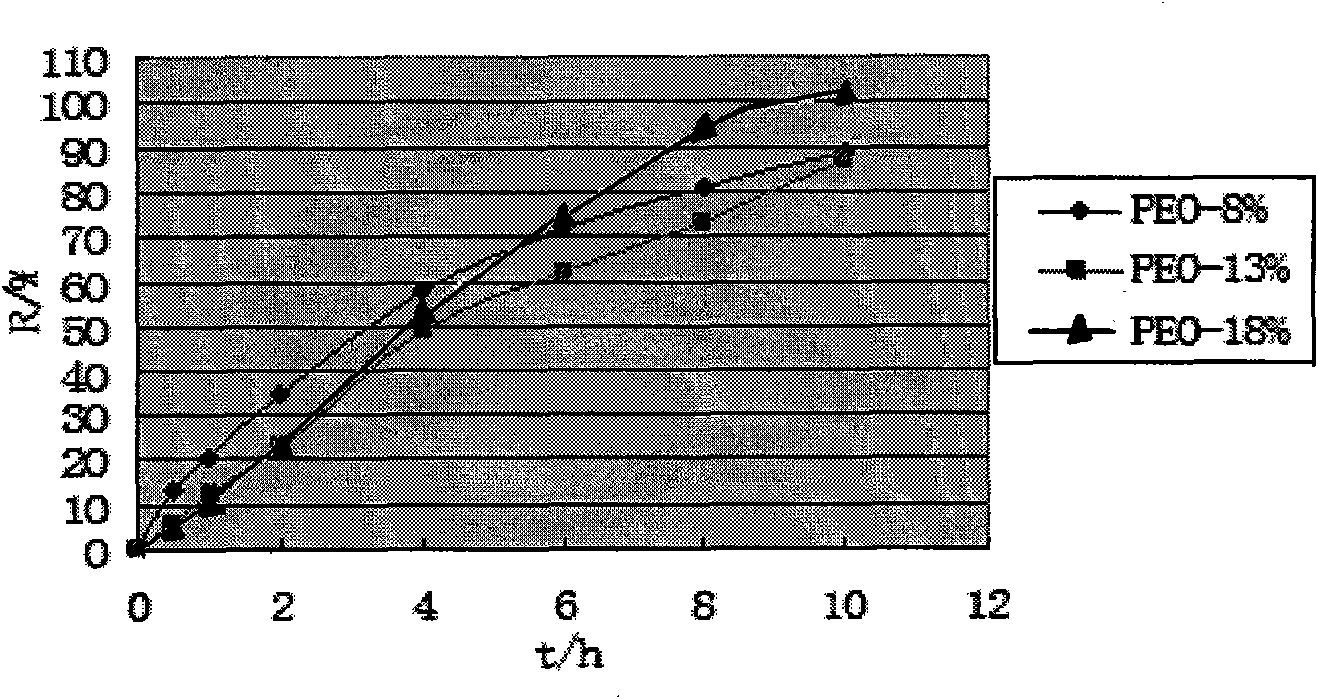
[0054] Take petracenin (187.5mg / tablet) and osmotically active substance mannitol (150mg / tablet), PEO (average molecular weight 5.5 million, its consumption is 45% of tablet core weight), PVP k30 (90mg / tablet) and mix well, use 95% ethanol solution as binder, make soft material, granulate through 20 mesh sieve, dry at 40°C for 4h, granulate with 20 mesh sieve, add 0.5% magnesium stearate for lubrication agent, and compress the tablet core (each containing 187.5 mg of petogenin) with a single-punch tablet press. Use the acetone:ethanol (95:5) solution of cellulose diacetate and PEG1500 as the coating solution, the coating temperature is 40-50°C, and the rotation speed is 30r min -1 , the weight gain of the coating was 10%, and it was left to solidify naturally, and then dried in an oven at 40° C. for 12 hours to remove...
Embodiment 2
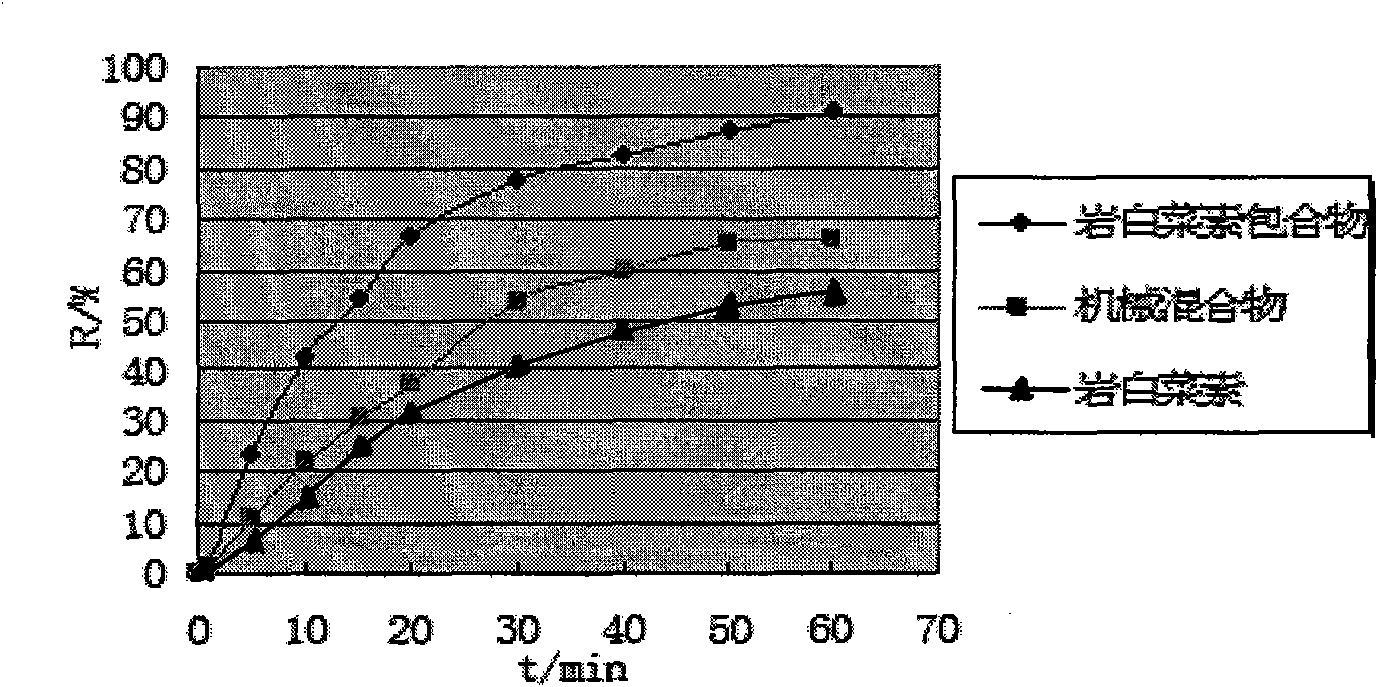
[0059] Example 2 Forming Process of Petgenin Inclusion Complex Osmotic Pump Tablets
[0060] 1 Experimental equipment and reagents
[0061] 1.1 Instrument
[0062] RCZ-5A Intelligent Drug Release Tester (Tianjin University Precision Instrument Factory); UV-1102 UV-Vis Spectrophotometer (Shanghai Tianmei Scientific Instrument Co., Ltd.); BY300A Small Coating Machine (Shanghai Huanghai Drug Testing Instrument Factory); TDP single punch Tablet machine (Shanghai No. 1 Pharmaceutical Machinery Factory); electronic balance (F1004, Shanghai Jinke balance); digital constant temperature water bath (HH-2, Guohua Electric Co., Ltd.); stirrer (JJ-11 booster electric stirrer , Jintan City Medical Instrument Factory).
[0063] 1.2 Reagent
[0064] Brutin reference substance (batch number: 111532-200202, National Institute for the Control of Pharmaceutical and Biological Products); Brutin raw material drug (batch number: 20070311, content 99.1%, Xichang Jiexiang Pharmaceutical Raw Materia...
Embodiment 3
[0121] Embodiment 3 Petrogenin Solid Dispersion Osmotic Pump Tablet Forming Process
[0122] 1 Experimental equipment and reagents
[0123] 1.1 Instrument
[0124] RCZ-5A Intelligent Drug Dissolution Apparatus (Tianjin University Precision Instrument Factory), UV-1102 UV-Vis Spectrophotometer (Shanghai Tianmei Scientific Instrument Co., Ltd.), BY-300A Small Coating Machine (Shanghai Huanghai Drug Inspection Instrument Co., Ltd.), TDP Single punch tablet machine (Shanghai No.1 Pharmaceutical Machinery Factory), electronic balance (F1004, Shanghai Jinke Tianping), HH-S constant temperature water bath (Jiangsu Guosheng Experimental Instrument Factory), ultrasonic cleaner (Tianjin Autosaiens Instrument Co., Ltd.), JJ-11 booster electric stirrer (Jintan Medical Instrument Factory).
[0125] 1.2 Reagent
[0126] Brutin raw material drug (content 99.1%, Xichang Jiexiang Pharmaceutical Raw Materials Co., Ltd., batch number: 20070311), Brutin reference substance (for content determi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com