Method for synthesizing formoononetin
A technology of formononetin and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of high cost, difficult to achieve industrial production, high production cost, etc., and achieve the effects of reducing production cost, reducing total cost and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
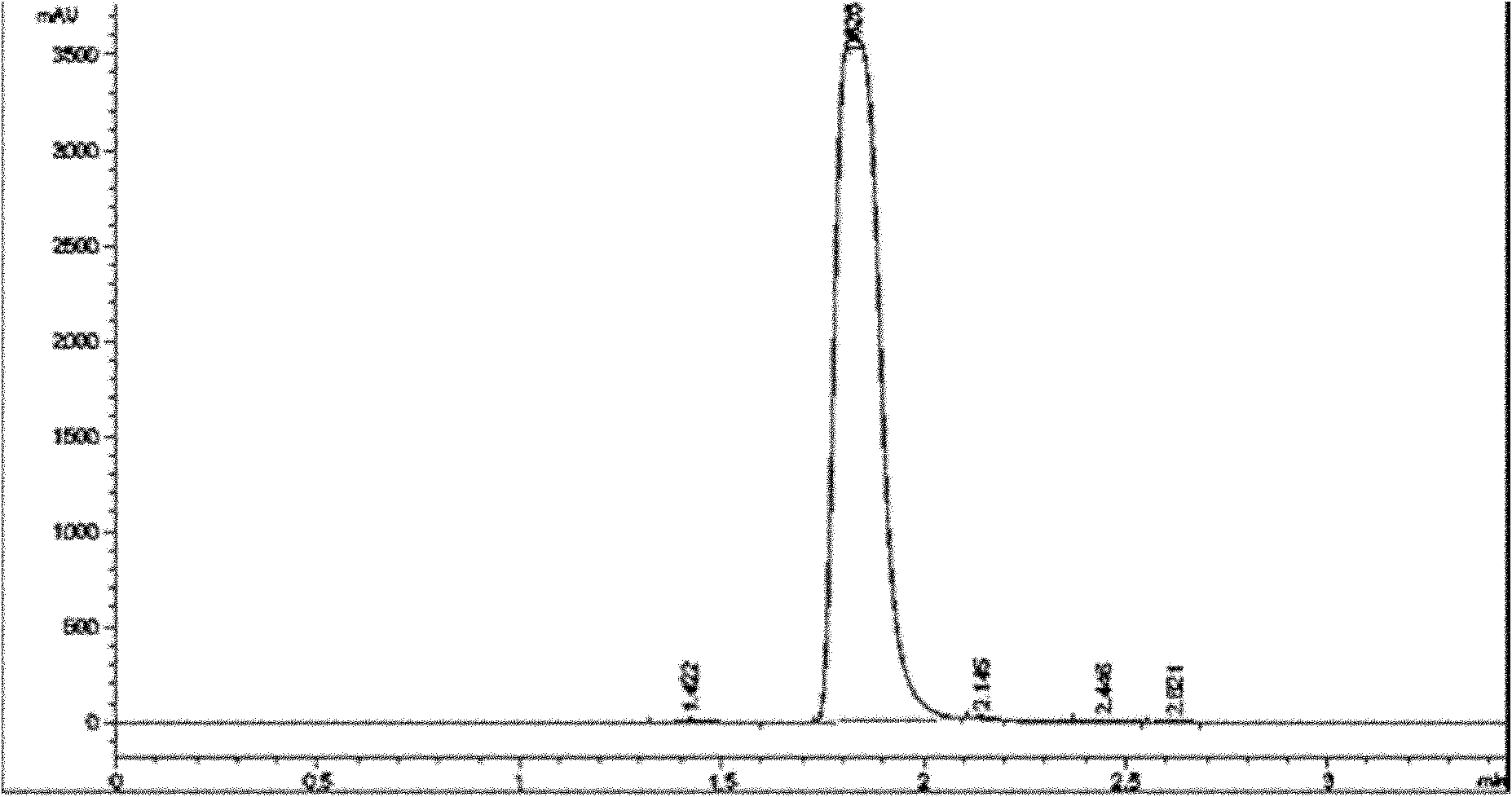
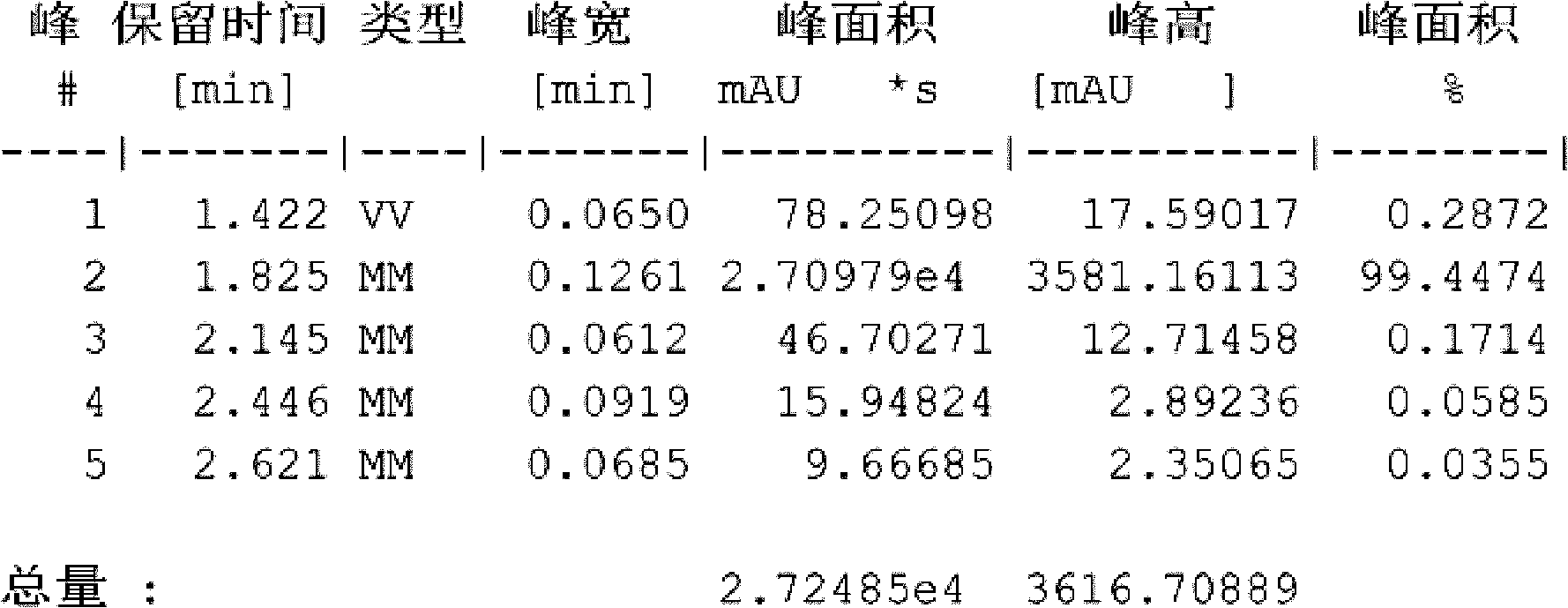
Image
Examples
Embodiment 1
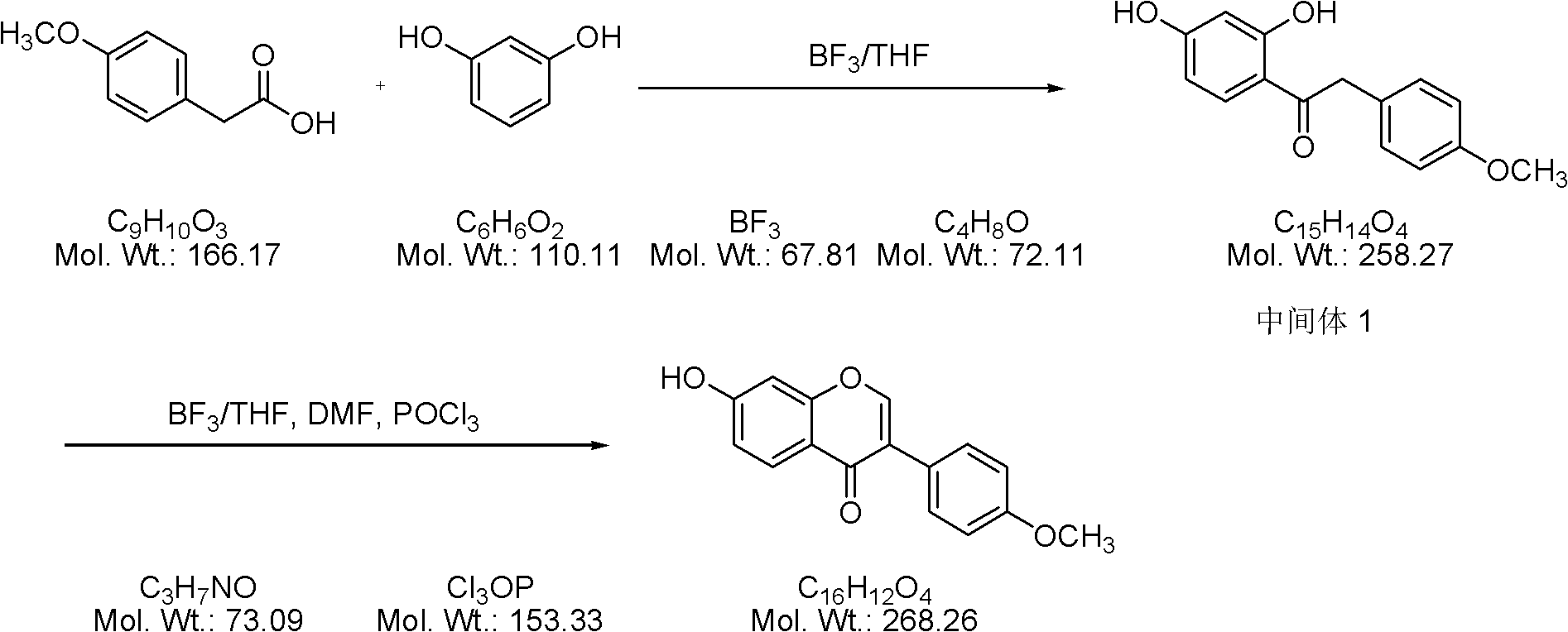
[0025] 1) 109 kilograms of p-methoxyphenylacetic acid and 80 kilograms of resorcinol are added to a 2000-liter reaction kettle, then 415 kilograms of boron trifluoride-tetrahydrofuran solution is added, and the temperature of the mixed solution is raised to 45° C. Stirring and reacting at this temperature for 3 hours;
[0026] 2) the temperature of the reaction solution after the above reaction is down to room temperature, add 800 kg of water and continue to stir the reaction for 10 hours, then stop stirring, leave standstill, and filter to obtain 169 kg of filtrate, and then use 500 kg of water-methanol for the filtrate A mixed solvent (with a water content of 15%) was recrystallized and filtered to obtain 166 kg of Intermediate 1 with a yield of 98%;
[0027] 3) Mix 166 kg of the above-mentioned intermediate with 350 kg of boron trifluoride-tetrahydrofuran solution in a 2000 liter reactor, then add 1000 kg of N,N-dimethylformamide dropwise at a temperature of 13°C to obtain ...
Embodiment 2
[0032]1) Add 166 grams of p-methoxyphenylacetic acid and 122 grams of resorcinol to a 2000 ml four-necked flask, then add 750 grams of boron trifluoride-tetrahydrofuran solution, and raise the temperature of the mixture to about 45°C , stirring and reacting at this temperature for 3 hours;
[0033] 2) The temperature of the reaction solution after the above reaction is down to room temperature, add 1300 grams of water and continue to stir the reaction for 10 hours, then stop stirring, leave standstill, filter to obtain 255 grams of filtrate, and then use the filtrate with 800 grams of water- Methanol mixed solvent (water content is 20%) is recrystallized, obtains 249 grams of intermediates, and the productive rate is 96%;
[0034] 3) Mix 249 grams of the above-mentioned intermediate with 500 grams of boron trifluoride-tetrahydrofuran solution in a 3000 ml four-necked flask, and then add 1700 grams of N,N-dimethylformamide dropwise at a temperature of 13°C to obtain Solution A...
Embodiment 3
[0039] 1) Add 166 grams of p-methoxyphenylacetic acid and 122 grams of resorcinol to a 2000 ml four-necked flask, then add 900 grams of boron trifluoride-tetrahydrofuran solution, and raise the temperature of the mixture to about 45°C , stirring and reacting at this temperature for 3 hours;
[0040] 2) The temperature of the reaction solution after the above reaction is down to room temperature, add 1500 grams of water and continue to stir the reaction for 10 hours, then stop stirring, leave standstill, filter to obtain 250 grams of filtrate, and then use the filtrate with 1000 grams of water- Methanol mixed solvent (water content is 25%) carries out recrystallization, obtains 237 grams of intermediates by filtration, and yield is 92%;
[0041] 3) Mix 237 grams of the above-mentioned intermediate with 500 grams of boron trifluoride-tetrahydrofuran solution in a 3000 ml four-necked flask, and then add 1800 grams of N,N-dimethylformamide dropwise at a temperature of 13°C to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com