Method for preparing chromic anhydride from potassium chromate
A technology of chromic anhydride and potassium chromate, applied in the field of inorganic salt production, can solve the problems of easy decomposition, reduce product yield, separation difficulty, etc., and achieve the effects of expanding product varieties, high product quality, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
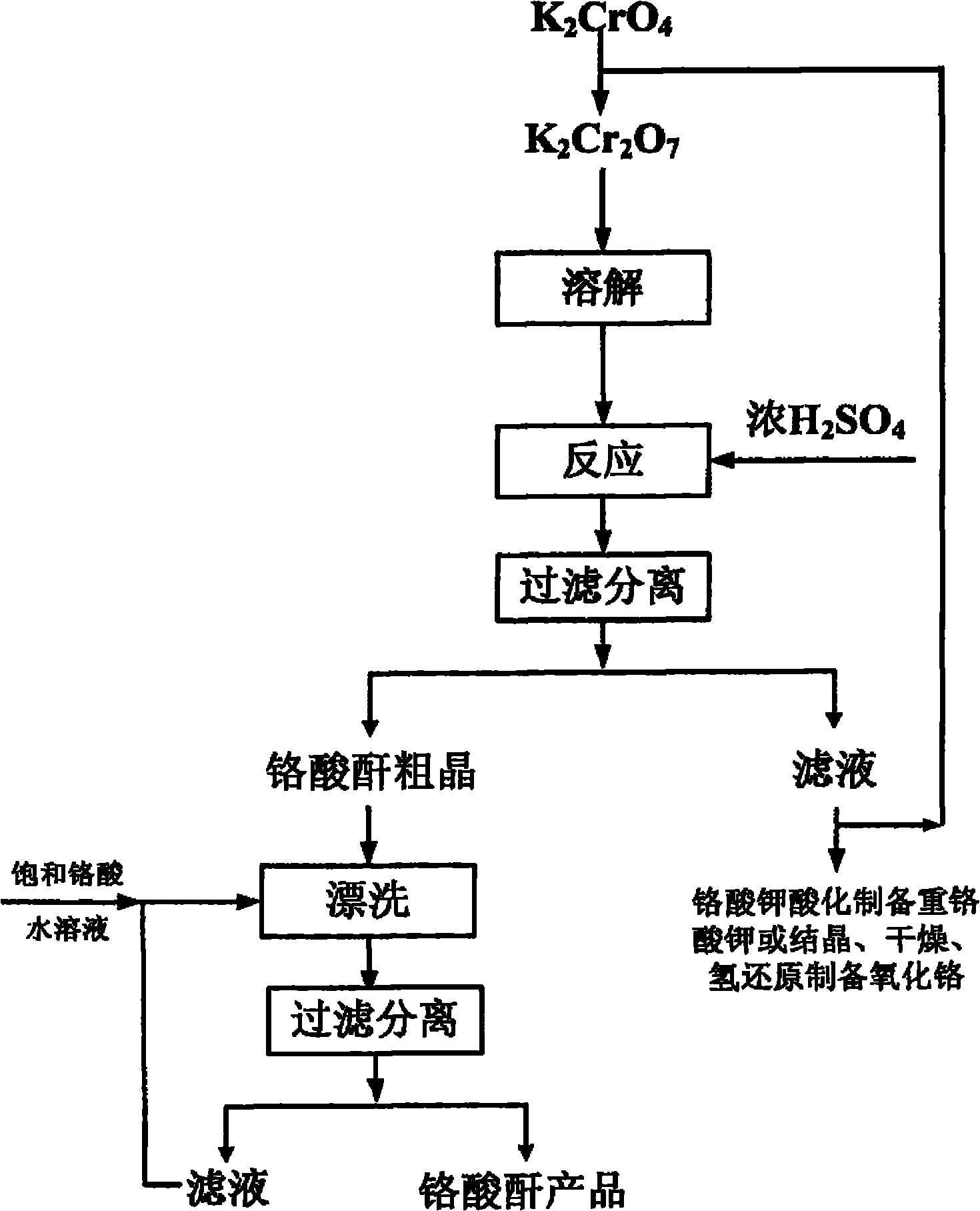
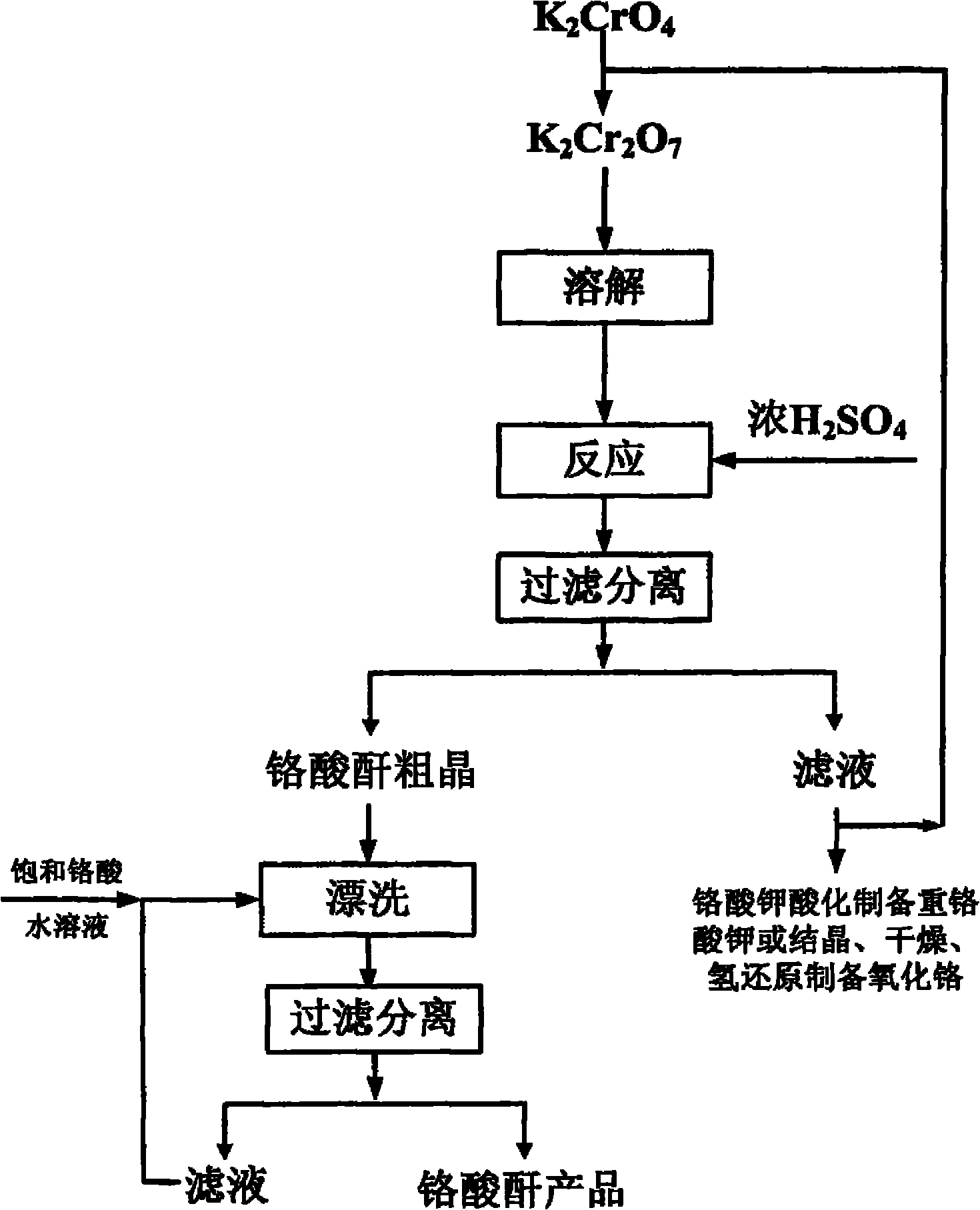
[0030] Dissolve and acidify potassium chromate to prepare a saturated potassium dichromate solution at 70°C. Slowly add the required amount of concentrated sulfuric acid to the potassium dichromate solution at a molar ratio of concentrated sulfuric acid to potassium dichromate of 7:1. The reaction was fully stirred for 1 h, and then filtered at 85°C to obtain crude chromic anhydride crystals. 40% of the obtained filtrate was returned to the acidified potassium chromate solution, and the obtained crystals were washed with a saturated chromic anhydride solution at 85° C., dried in an oven at 101° C. for 2 hours, and packaged after cooling to obtain a chromic anhydride product with higher purity. .
Embodiment 2
[0032] Dissolve and acidify potassium chromate to prepare a saturated potassium chromate solution at 90°C. Slowly add the required amount of concentrated sulfuric acid to the potassium dichromate solution at a molar ratio of concentrated sulfuric acid to potassium dichromate of 5:1. The reaction was fully stirred for 2h, and then filtered at a reaction temperature of 90°C to obtain crude chromic anhydride crystals. 50% of the obtained filtrate was returned to the acidified potassium chromate solution, and the obtained crystals were washed with a saturated chromic anhydride solution at 95°C, dried in an oven at 101°C for 3 hours, and packaged after cooling to obtain a chromic anhydride product with higher purity .
Embodiment 3
[0034] Dissolve potassium chromate and acidify to prepare a saturated potassium chromate solution at 90°C. Slowly add the required amount of concentrated sulfuric acid to the potassium dichromate solution at a molar ratio of concentrated sulfuric acid to potassium dichromate of 1:1. The mixture was fully stirred for 0.5 h, heated up and then filtered at 95°C to obtain crude chromic anhydride crystals. 60% of the obtained filtrate was returned to the acidified potassium chromate solution, and the obtained crystals were washed with a saturated chromic anhydride solution at 95°C, dried in an oven at 101°C for 3 hours, and packaged after cooling to obtain a chromic anhydride product with higher purity .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com