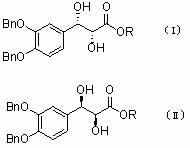
Asymmetric synthesis method of 3-(3,4-dihydroxy phenyl)-2-hydracrylate
A technology of hydroxypropionate and dihydroxyphenyl is applied in the field of medicine and health to achieve the effect of high optical purity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of 3,4-dibenzyloxybenzaldehyde (1)
[0029] Dissolve 2.76g (20 mmol) of 3,4-dihydroxybenzaldehyde in 40 mL of anhydrous DMF, add 13.8 g of anhydrous potassium carbonate and 6.32 g (50 mmol) of benzyl chloride in sequence, and react the mixture at 75°C for 4 h. After filtering and concentrating the filtrate, the resulting oil was poured into 50 mL of distilled water with stirring, resulting in a large amount of solid, which was filtered and dried to obtain 5.78 g of a pale yellow solid, with a yield of 90.8%. m.p. 90°C-91°C.
Embodiment 2
[0030] Example 2 E -Synthesis of 3-(3,4-dibenzyloxyphenyl)acrylic acid (2)
[0031] Add 6.0 g (18.9 mmol) of 3,4-dibenzyloxybenzaldehyde and 5.88 g (57 mmol) of malonic acid into a three-necked flask, add 30 mL of 1,4-dioxane, and then add a catalytic amount of Pyridine and piperidine were reacted under reflux for 5h, TLC showed that the starting material disappeared, and cooled to room temperature. The reaction solution was poured into ice water, concentrated HCl was added, and a large amount of white solid was precipitated, which was filtered and washed with water. The crude product was recrystallized with 95% ethanol to obtain 5.7 g of white solid, with a yield of 85%. m.p. 201-202°C. 1 H NMR (500 MHz, CDCl 3 ) , δ 7.67-7.63(d, J =15.85Hz, 1H),7.46-7.43(m,4H),7.39-7.36(m,4H),7.33-7.30(m,2H),7.14-7.13(d, J =1.95Hz,1H),7.10-7.08(dd, J 1 =1.9Hz, J 2 =2.0Hz, 1H), 6.94-6.92 (d, J =8.35Hz, 1H),6.25-6.22(d, J =15.85Hz, 1H), 5.21-5.19 (d, J =10.6Hz, 4H); ...
Embodiment 3
[0032] Example 3 E -Synthesis of isopropyl 3-(3,4-dibenzyloxyphenyl)acrylate (3)
[0033] Will Z - 3.0 g (8.3 mmol) of 3-(3,4-dibenzyloxyphenyl) acrylic acid and 50 mL of isopropanol were added to the flask, and 1 mL of concentrated H 2 SO 4 , refluxed for 15h, TLC showed that the raw material disappeared, placed at room temperature, slowly added 50 mL saturated NaHCO 3 , extracted with toluene (3×30 mL), separated the organic layer, anhydrous MgSO 4 Dry, filter, and remove the solvent under reduced pressure to give a white solid. Recrystallized with ethyl acetate: n-hexane = 1:3 to obtain 2.7 g of the product with a yield of 80%. m.p.113.7℃-114.5℃. 1H NMR (400 MHz, CDCl 3 ) ,δ 7.59-7.55 (d, J =16Hz, 1H),7.49-7.45(m,5H),7.41-7.35(m,5H),7.14(s,1H),7.10-7.08(d, J =8.4Hz, 1H),6.95-6.93(d, J = 8.4Hz, 1H),6.27-6.23(d, J =15.6Hz, 1H), 5.22-5.20(d, J =8.8Hz, 4H),5.16-5.11 (m,1H), 1.33-1.32(d, J =6.4Hz, 6H); 13 C NMR (125 MHz, CDCl 3 ), δ for MS C. 22 26 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com