Beta-cyclodextrin derivative, preparation method and application thereof
A technology for cyclodextrin and derivatives, applied in the field of cyclodextrin derivatives and their preparation, can solve the problems of low chemical activity, complex environment, harmfulness and the like, and achieve a simple preparation process, convenient post-processing and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Physical measurement means and used chemical materials
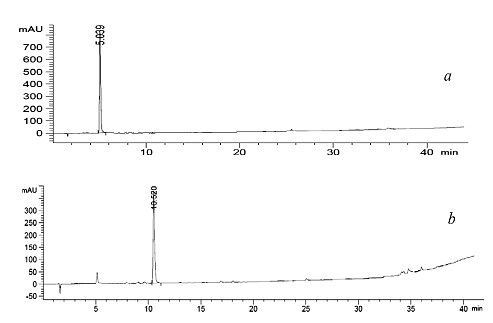
[0026] The high-performance liquid chromatography used an Agilent 1200 high-performance liquid chromatography system with a UV detector, and the separation column was a Thermo Hypersil-Keystone AQUASILC18 column (4.6 mm×150 mm). The gradient elution of high performance liquid chromatography is completed within 40 minutes, and MeCN and H in the eluent 2 The ratio of O was changed linearly from 0:100 to 100:0 with a flow rate of 1.0 mL / min.
[0027] Infrared detection uses Bruker IFS66V FT-IR spectrophotometer to measure by KBr tablet method.
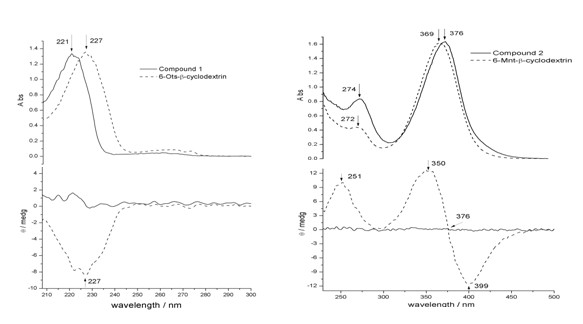
[0028] UV spectra were recorded with a Shimadzu UV-3100 spectrometer.
[0029] all 13 C and 1 The H-NMR spectra were all recorded on a Bruker AVANCE-500 spectrometer, the measurement temperature was 15°C, and the solvent was deuterated-dimethyl sulfoxide or heavy water.
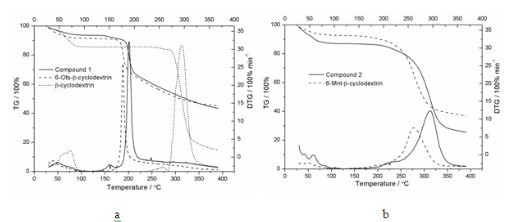
[0030] The thermogravimetric analysis curve was recorded on the SDT-2960 thermal analyz...
Embodiment 2
[0032] Example 2 Synthesis of Intermediate 2-Ots-β-CD
[0033]β-cyclodextrin (11.5g, 10mmol) was mixed with Ts 2 O (4.9g, 15mmol) was mixed in 250ml of water, and stirred at room temperature for 2h (stirring speed was 200-400 rpm); 50ml of sodium hydroxide solution with a mass volume ratio of 10% was added, and after 1h of reaction, sand Core funnel filtration to remove unreacted Ts 2 O; add dilute hydrochloric acid solution with a concentration of about 0.5-1 mol / L to adjust to pH 3-4, and use a reduced-pressure rotary evaporation method to concentrate the solution to 50ml; control the temperature at 4°C, and cool it statically for more than 12 hours. Collect the precipitate to obtain 2-Ots - Crude β-CD; the crude product was washed with acetone and dried in vacuo to obtain the intermediate 2-Ots-β-CD as a white powder (7.4 g, 51.4%). To characterize 2-Ots-β-CD, the specific data are as follows:
[0034] Elemental analysis results: C, 42.54; H, 5.84. According to formula ...
Embodiment 3
[0039] Example 3 Synthesis of 2-Mnt-β-CD
[0040] After dissolving 3g of 2-Ots-β-CD in 5ml of water, add this solution to 10ml of an aqueous solution containing 1.2g of disodium maleicitrocyanodithionate, control the temperature at 65°C, and stir for 1 hour; cool the solution After reaching room temperature and filtering with filter paper, the filtrate was concentrated to 2-5ml by rotary evaporation under reduced pressure, and then added dropwise at a rate of 0.5 drops / second to 400ml of absolute ethanol that was vigorously stirred (stirring rate was about 500 rpm) to obtain a precipitate that was It is the crude product of 2-Mnt-β-CD; dissolve the crude product in 25ml of water, filter it through filter paper, concentrate, and then add it dropwise to 500ml of absolute ethanol with vigorous stirring (stirring rate is about 500 rpm); dropwise After standing still for more than 12 hours, the final product was precipitated from the ethanol solution; filtered through filter paper ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com