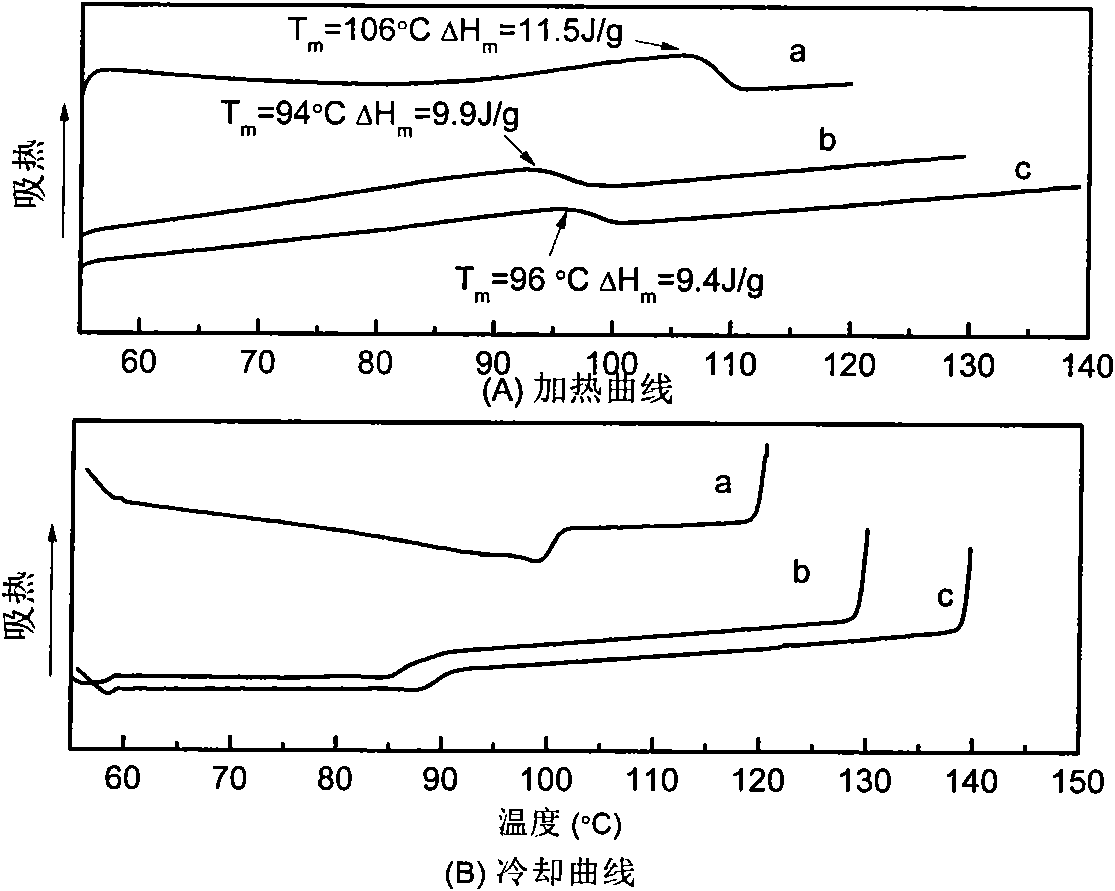
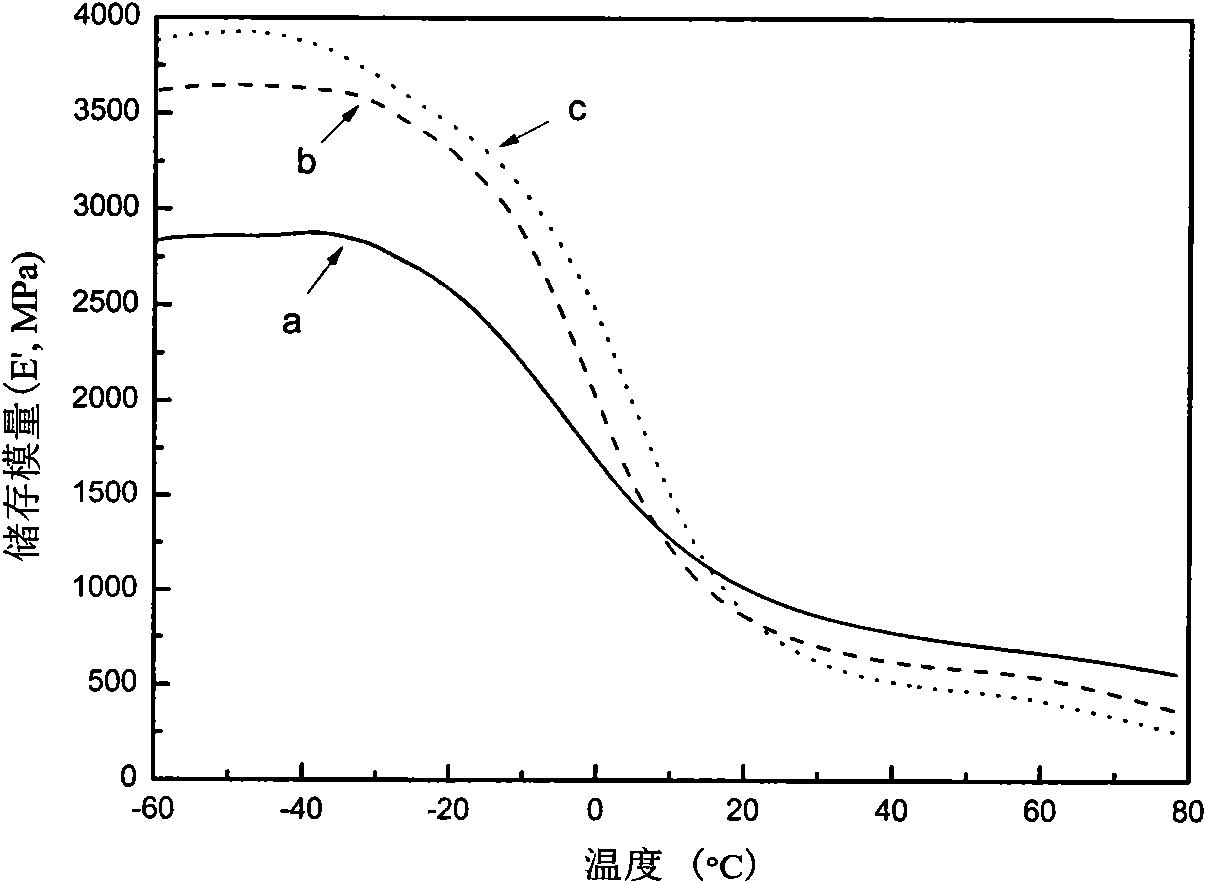
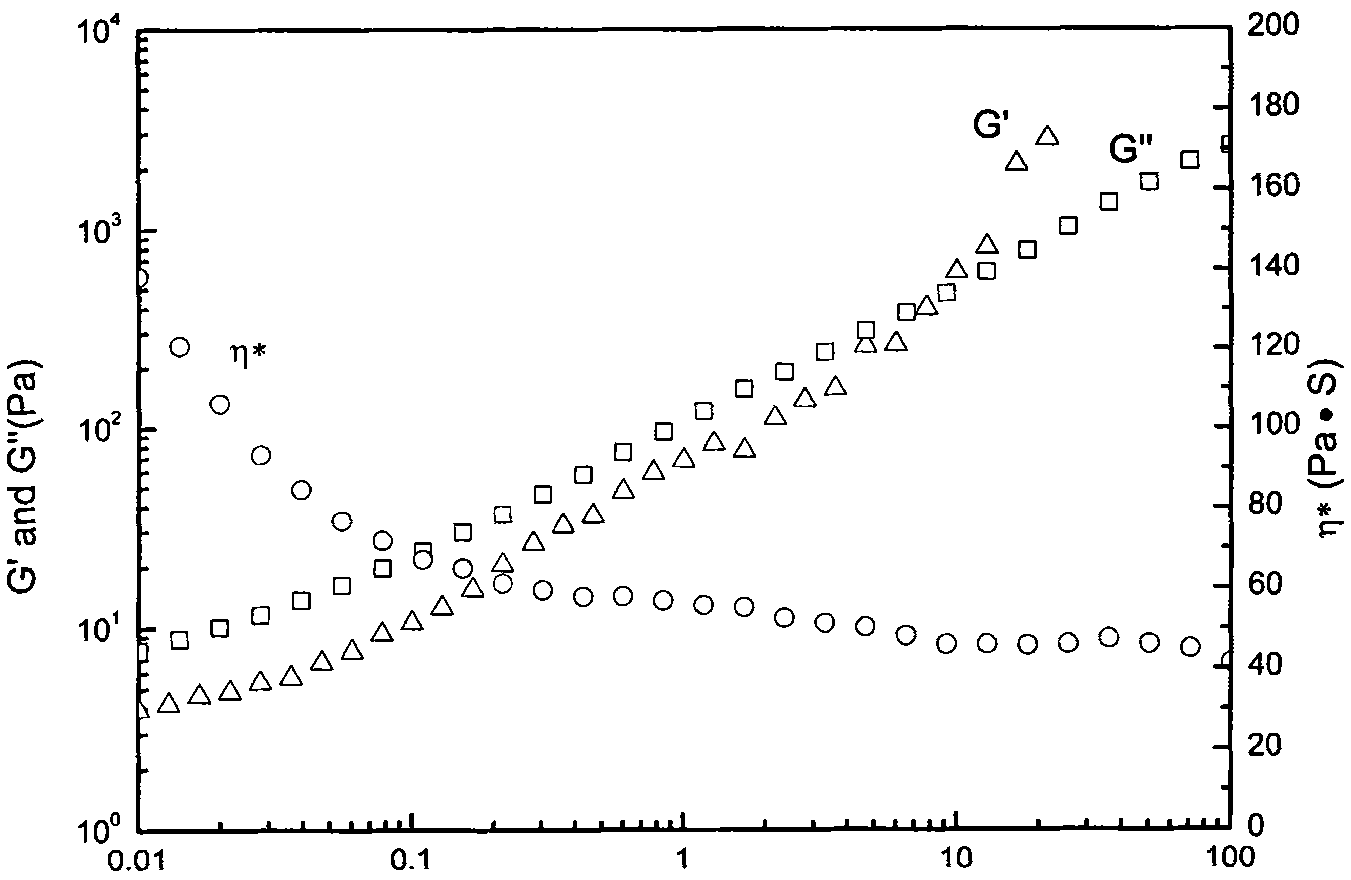
Thermal restoring net-structured hydrogen bond supermolecule elastomeric polymer and preparation method thereof
A network structure and supramolecular technology, applied in the field of oligomers constituting the polymer, to achieve good thermal recovery, low cost, and large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Add ethylenediamine to the dimerized fatty acid A, under 200mL / min nitrogen atmosphere, magnetically stir, heat the oil bath to 180°C, condense and reflux for 8 hours, the ratio of dimerized fatty acid A to ethylenediamine is 1:1.2 ;
[0036] (2) Remove the condensation reflux device, heat at 160°C oil temperature for 7h, and at the same time pass 500mL / min nitrogen gas to take away the remaining unreacted ethylenediamine and water.
[0037] (3) Granulation and impurity removal: After the above reaction is stopped, pour the product on the release paper while it is hot. After the material was cooled to room temperature, the material was granulated into pieces with a thickness of 1 mm, soaked in a mixed solution of methanol / water (2:1 by volume) for 72 hours, and dried in a vacuum oven at 60°C for 72 hours after soaking to obtain final sample.
Embodiment 2
[0039](1) Add ethylenediamine to the dimerized fatty acid A, under 200mL / min nitrogen atmosphere, magnetically stir, heat the oil bath to 180°C, condense and reflux for 8 hours, the ratio of dimerized fatty acid A to ethylenediamine is 1:1.2 ;
[0040] (2) Remove the condensation reflux device, heat at 160°C oil temperature for 7h, and at the same time pass 500mL / min nitrogen gas to take away the remaining unreacted ethylenediamine and water.
[0041] (3) Synthesis of monoisocyanate-terminated amide oligomers: After removing diamine monomers, add a certain amount of terephthalic acid monoisocyanate, and heat the reaction mixture to 180°C in a nitrogen atmosphere of 200mL / min, and react for 4h , The ratio of dimer fatty acid to terephthalic acid monoisocyanate is 1:0.2.
[0042] (4) Granulation and impurity removal: After the above reaction is stopped, pour the product on the release paper while it is hot. After the material was cooled to room temperature, the material was gr...
Embodiment 3
[0044] (1) Add ethylenediamine to dimerized fatty acid B, under 200mL / min nitrogen atmosphere, magnetically stir, heat up the oil bath to 180°C, condense and reflux for 8 hours, the ratio of dimerized fatty acid A to ethylenediamine is 1:1.2 ;
[0045] (2) Remove the condensation reflux device, heat at 160°C oil temperature for 7h, and at the same time pass 500mL / min nitrogen gas to take away the remaining unreacted ethylenediamine and water.
[0046] (3) Synthesis of monoisocyanate-terminated amide oligomers: After removing diamine monomers, add a certain amount of terephthalic acid monoisocyanate, and heat the reaction mixture to 180°C in a nitrogen atmosphere of 200mL / min, and react for 4h , The ratio of dimer fatty acid to terephthalic acid monoisocyanate is 1:0.2.
[0047] (4) Granulation and impurity removal: After the above reaction is stopped, pour the product on the release paper while it is hot. After the material was cooled to room temperature, the material was gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com