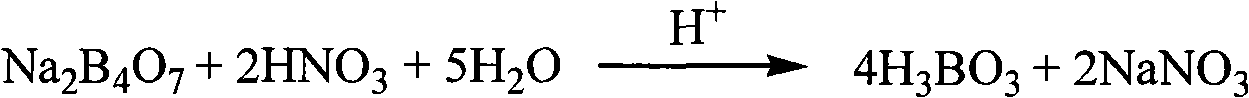Method for testing content of boron trioxide in glass containing zinc and lead
A technology of diboron trioxide and testing methods, which is applied in the direction of analyzing materials through chemical reactions and material analysis through observing the impact on chemical indicators, and can solve problems affecting the accuracy of test results and interfering with measurement. Achieve the effect of clear discoloration point, easy to control, and eliminate the interference of gender elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
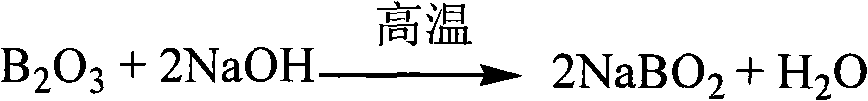
[0030] Accurately weigh 0.5g of glass (containing lead and zinc) sample, add 4g of sodium hydroxide to melt in a nickel crucible, soak the melt with hot water, add 2 drops of methyl red indicator, and neutralize with 1mol / L hydrochloric acid Until the system turns reddish, calcium carbonate is added until the system turns yellow, and the precipitate is filtered to obtain solution A.
[0031] Add 10 mL of 0.1 mol / L Ca-EDTA solution to solution A, adjust the solution to reddish with 0.1 mol / L hydrochloric acid, and obtain solution B.
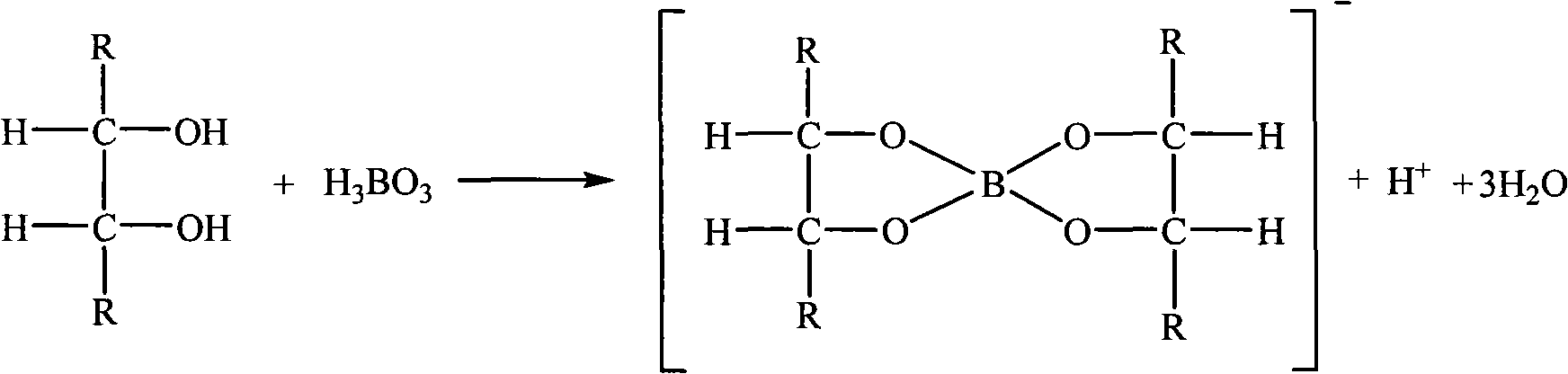
[0032] Titrate solution B with 0.1mol / L sodium hydroxide standard solution to turn yellow (do not record the reading), add 2g mannitol, 10 drops of 1:1 bromothymol blue and phenol red mixed indicator, and oxidize with 0.1mol / L hydroxide Titrate the sodium standard solution to purple; then add 2g mannitol, the purple color disappears, continue to titrate with 0.1mol / L sodium hydroxide standard solution, and repeat this, until the purple color of th...
Embodiment 2
[0040] Accurately weigh 0.5g glass sample (containing lead and zinc), add about 5g potassium hydroxide and melt it in a nickel crucible. Extract the molten material with hot water, add 2 drops of methyl red indicator dropwise, neutralize with 2mol / L nitric acid until the system turns reddish, add sodium carbonate until the system turns yellow, filter the precipitate to obtain solution A.
[0041] Add 15 mL of 0.1 mol / L Ca-EDTA solution to solution A, adjust the solution to reddish with 0.2 mol / L hydrochloric acid, and obtain solution B.
[0042] Neutralize solution B with 0.05mol / L sodium hydroxide until the solution turns yellow, add 2g of mannitol, 10 drops of 1:0.5 bromothymol blue and phenol red mixed indicator, and titrate with 0.1mol / L sodium hydroxide standard solution to purple; then add 2g of mannitol, purple disappears, continue to titrate with 0.1mol / L sodium hydroxide standard solution, and so on, until the purple color of the test solution does not disappear after...
Embodiment 3
[0047] Accurately weigh 0.5g glass sample (containing lead and zinc), add about 5g potassium hydroxide and melt it in a nickel crucible. Extract the molten material with hot water, add 2 drops of methyl red indicator dropwise, neutralize with 5mol / L hydrochloric acid until the system turns reddish, add potassium carbonate until the system turns yellow, filter the precipitate, and obtain solution A.
[0048] Add 25 mL of 0.1 mol / LCa-EDTA solution to solution A, adjust the solution to reddish with 0.5 mol / L hydrochloric acid, and obtain solution B.
[0049] Neutralize solution B with 0.11mol / L sodium hydroxide solution until the system turns yellow, add 2g mannitol, 10 drops of 1:1.5 bromothymol blue and phenol red mixed indicator, use 0.2mol / L sodium hydroxide standard The solution is titrated to purple; add 2g of mannitol again, the purple color disappears, continue to titrate with 0.2mol / L sodium hydroxide standard solution, and so on, until the purple color of the test solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com