Hydrophylic phenylboric acid functional porous integral material, preparation method and application thereof
A monolithic material, phenylboronic acid technology, applied in chemical instruments and methods, inorganic chemistry, alkali metal compounds, etc., can solve the problem of sample denaturation, achieve the effect of low production cost, good repeatability, and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
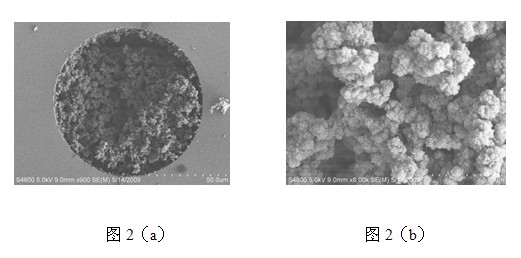
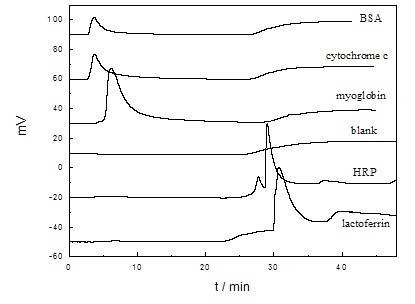
Image
Examples
Embodiment 1
[0014] Example 1 Preparation of bulk hydrophilic phenylboronic acid functionalized porous monolith
[0015] (1) Take a suitable size centrifuge tube, dissolve 60mg SPBA and 120mg MBAA in 340uL DMSO solution, add 250mg dodecanol, 4mg AIBN, vortex and sonicate for 15 minutes, seal it, and put it in a constant temperature water bath React at 75°C for 12 hours;
[0016] (2) Then take out the whole material, cut it into small pieces, put it into a Soxhlet extractor, add methanol at a temperature of 110°C, and extract for 24 hours to remove unreacted monomers and cross-linking agents;
[0017] (3) Put the above materials in a vacuum drying oven and dry them at 100°C for 12 hours to obtain a hydrophilic phenylboronic acid functional monolith;
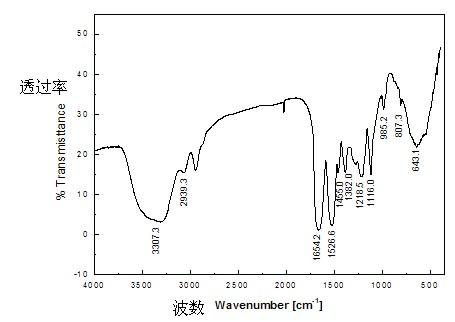
[0018] (4) Grind the above materials into granules, mix them with potassium bromide crystals, and grind them together into powders less than 200 meshes. After tableting, use Fourier transform infrared spectroscopy to characterize them. Sulfon...
Embodiment 2
[0019] Example 2: Preparation of hydrophilic phenylboronic acid functionalized porous monolith in capillary
[0020] Hydrophilic phenylboronic acid functionalized porous monoliths were synthesized in capillaries with different inner diameters (25mm, 75mm, 100mm, 150mm, 250mm):
[0021] (1) For the pretreatment of the capillary, first rinse the capillary empty column with 0.1M NaOH solution for 1 hour, then rinse the capillary with deionized water until the pH value of the effluent liquid is 7.0, then rinse the capillary with 0.1M HCl solution for 2 hours, and then use The capillary was rinsed with ionized water until the pH of the effluent liquid was 7.0, and then the capillary was rinsed with methanol solution for 30 minutes and dried with nitrogen. A mixture of methanol and methacryloxypropyl-trimethoxysilane was injected into the capillary. React at a temperature of 20°C to 70°C for 5-24 hours. Then rinse with methanol, and finally blow dry with nitrogen for use.
[0022...
Embodiment 3
[0027] Example 3: Preparation of hydrophilic phenylboronic acid functionalized porous monolithic material in conventional chromatographic column
[0028] Synthesis of hydrophilic phenylboronic acid-functionalized porous monoliths in conventional HPLC columns with different inner diameters:
[0029] (1) Cleaning of the conventional HPLC column, first, immerse the conventional HPLC column in deionized water, clean it ultrasonically for 1 hour, then replace it with methanol for ultrasonic cleaning 1, and dry it in an oven for 2 hours;
[0030] (2) Dissolve 150mg SPBA and 300mg MBAA in 850mL of DMSO solution, then add 625mg of dodecanol and 10mg of AIBN, vortex and sonicate for 15 minutes to obtain a polymerization solution;
[0031] (3) Add the prepared polymer solution carefully and slowly into the conventional HPLC column with a pipette gun;
[0032](4) Seal both ends of the processing conventional column with tips, put it in a water bath at 50-90°C, and react at a constant t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com