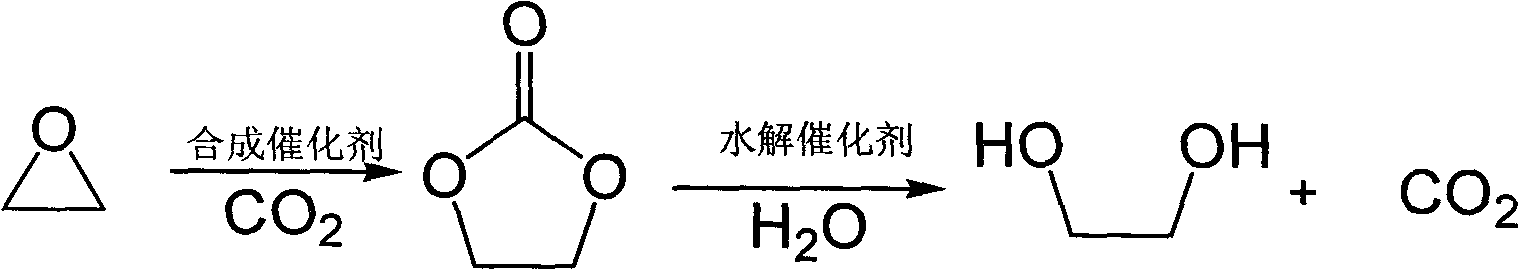
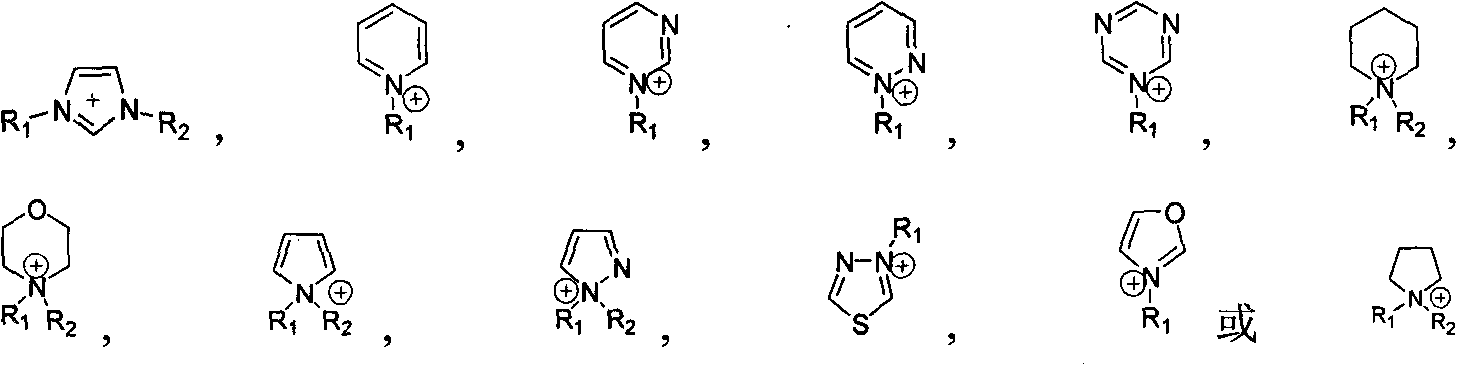
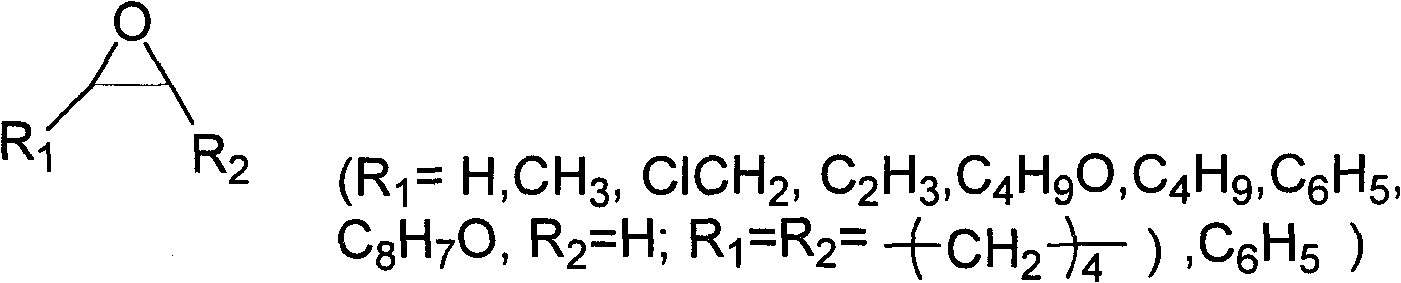Method for preparing dibasic alcohol
A diol and carbon dioxide technology, which is applied in the field of diol preparation, can solve problems such as difficulty in obtaining high-quality ethylene glycol, incomplete conversion of cyclic carbonates, and low selectivity of diols, and achieve effective The effect of utilization, high stability, and convenient circulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] In a 100 ml autoclave, add 0.05 mmol of metal salt zinc chloride, 0.5 mmol of ionic liquid 1-methyl-3-butylimidazolium bromide and 0.4 mmol of tetrabutylammonium bromide, and finally add 20 ml of epoxy Propane (1a), a closed reaction kettle, the temperature is controlled by a temperature controller to slowly rise to 100 ° C, maintain a reaction pressure of 1.5 MPa, react for 1 hour, cool to room temperature, unload the kettle, and separate the catalyst from the reaction liquid through vacuum distillation. The corresponding cyclic carbonate (2a) is then obtained. In the autoclave, 2.8 grams of polystyrene-supported N-methylimidazolium bicarbonate ionic liquid and 12.96 grams of water were successively added to generate 40.9 grams of propylene carbonate (2a). Close the reaction kettle, the temperature is controlled by the temperature controller to rise slowly to 140°C, keep the reaction pressure at 1.5MPa, react for 4 hours, cool to room temperature, collect t...
Embodiment 2
[0044] With embodiment 1, used synthetic reaction metal salt is zinc bromide, and the selectivity of propylene glycol (3a) is 99%, and yield is 99%;
Embodiment 3
[0046] Same as Example 1, the cocatalyst used in the synthesis reaction is 0.05 mmol of 1-methyl-3-butyl imidazolium chloride salt, N-butyl pyrimidine bromide salt, the selectivity of propylene glycol (3a) is 99%, and the yield is 99%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com