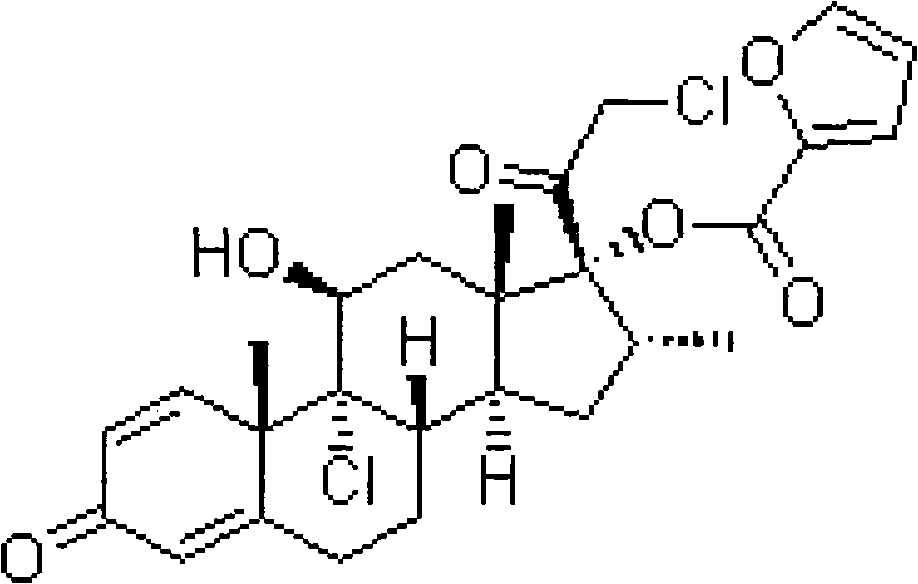
Nasal in-situ gel containing mometasone furoate and H1 receptor antagonist
A receptor antagonist, mometasone furoate technology, applied in the field of in situ gel, can solve the problems of undisclosed, decreased drug bioavailability, poor stability of nasal spray, etc., achieve good therapeutic effect and achieve sustained and stable release , Improve the effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] 1. Preparation of Levocetirizine Hydrochloride Cyclodextrin Inclusion Compound
[0042] Add 2.5g of β-cyclodextrin, add appropriate amount of distilled water and stir at 60°C until it is completely dissolved, add 0.5g of levocetirizine hydrochloride, continue stirring for 30 minutes, then pour it into an evaporating dish, evaporate to dryness in a water bath at 90°C, and dry in a desiccator , Grind finely to obtain levocetirizine hydrochloride / β-cyclodextrin inclusion compound, set aside.
[0043] 2. Preparation of desloratadine cyclodextrin inclusion aqueous solution
[0044] Dissolve 4 g of 2-hydroxypropyl-β-cyclodextrin in 25 ml of 80% ethanol at room temperature, add 0.2 g of declotadine, stir to dissolve, stir for another 30 minutes, filter through a microporous membrane (220 nm), add 10 mL of water for injection was stirred for 5 minutes, concentrated under reduced pressure, and the acetone was distilled off to obtain an aqueous solution of deslotetadine / 2-hydrox...
Embodiment 1
[0048] Embodiment 1 prepares ion-sensitive corticosteroid nasal in-situ gel, and the prescription is as follows (see Table 1):
[0049] Table 1 Prescription of ion-sensitive corticosteroid nasal in situ gel
[0050] content%
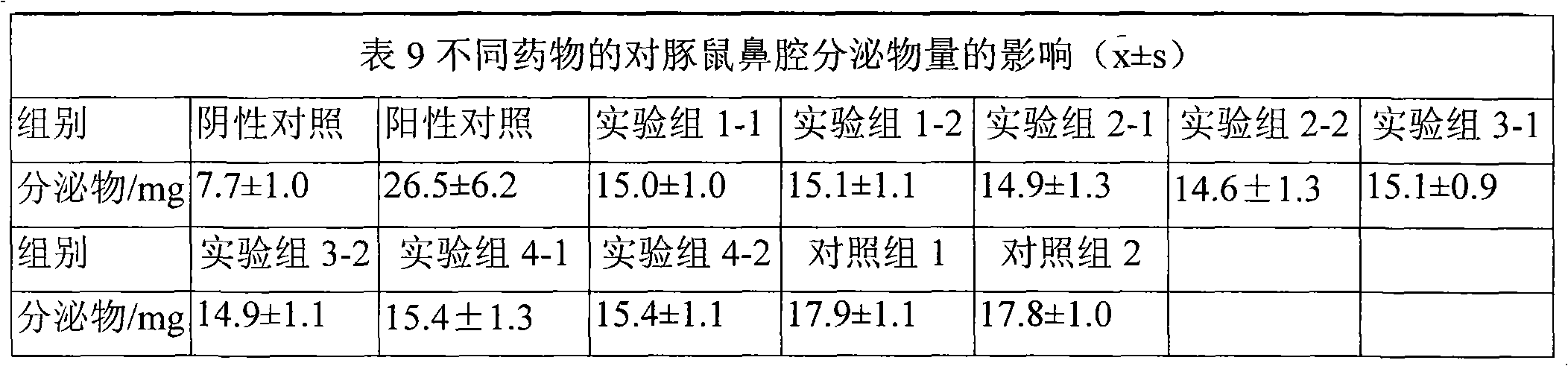
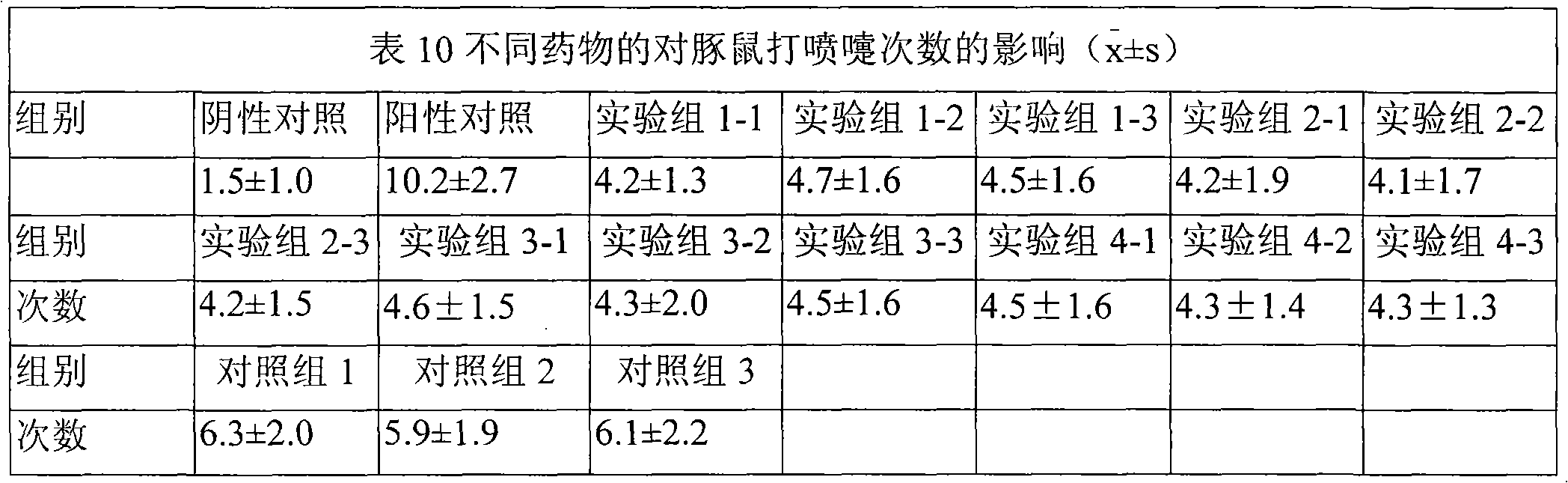
[0051] Preparation process: Place the above ion-sensitive polymer in an appropriate amount of deionized water, stir in a water bath at 80-100°C to disperse and dissolve, cool down to 4°C and place it in a refrigerator for 12 hours to obtain a clear solution (1). Example 1-1 to Example 1-4 Mix corticosteroid micropowder and H1 receptor antagonist or clathrate / clathate aqueous solution with other water-soluble excipients in water for injection to make suspension (2), Mix (1) and (2), adjust the pH to 6.2, and add water to the full amount. The rheological properties of the above five formulations were measured at room temperature (20° C.) using a Brookfield DV-m rotational viscometer. The viscosity of the above-mentioned prescriptions shows a do...
Embodiment 2
[0052] Embodiment 2 prepares temperature-sensitive corticosteroid nasal in-situ gel, and the prescription is as follows (see Table 2):
[0053] Table 1 Prescription of temperature-sensitive corticosteroid nasal in situ gel
[0054] content%
Example 2-1
Example 2-2
Example 2-3
Example 2-4
Active ingredient content%
mometasone furoate
Hydrate 0.05
mometasone furoate
0.05
mometasone furoate
0.1
mometasone furoate
Hydrate 0.1
H1 receptor antagonist content
%
Levocabaz Hydrochloride
Ting 0.05
0.1
Levocetirizine Hydrochloride
0.1
Levocabaz Hydrochloride
Ting 0.05
Poloxamer 407
20
35
12
Poloxamer 188
5
28
15
PEG (6000-11000)
2
Polyethylene oxide (PEO)
2.5
(HPMC)
2
1.5
s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com