Oxalic ester hydrogenated copper silicon catalyst and preparation method thereof
A technology of catalyst and oxalate, which is applied in molecular sieve catalysts, chemical instruments and methods, preparation of hydroxyl compounds, etc., to achieve the effects of easy large-scale production, high dispersion of copper, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
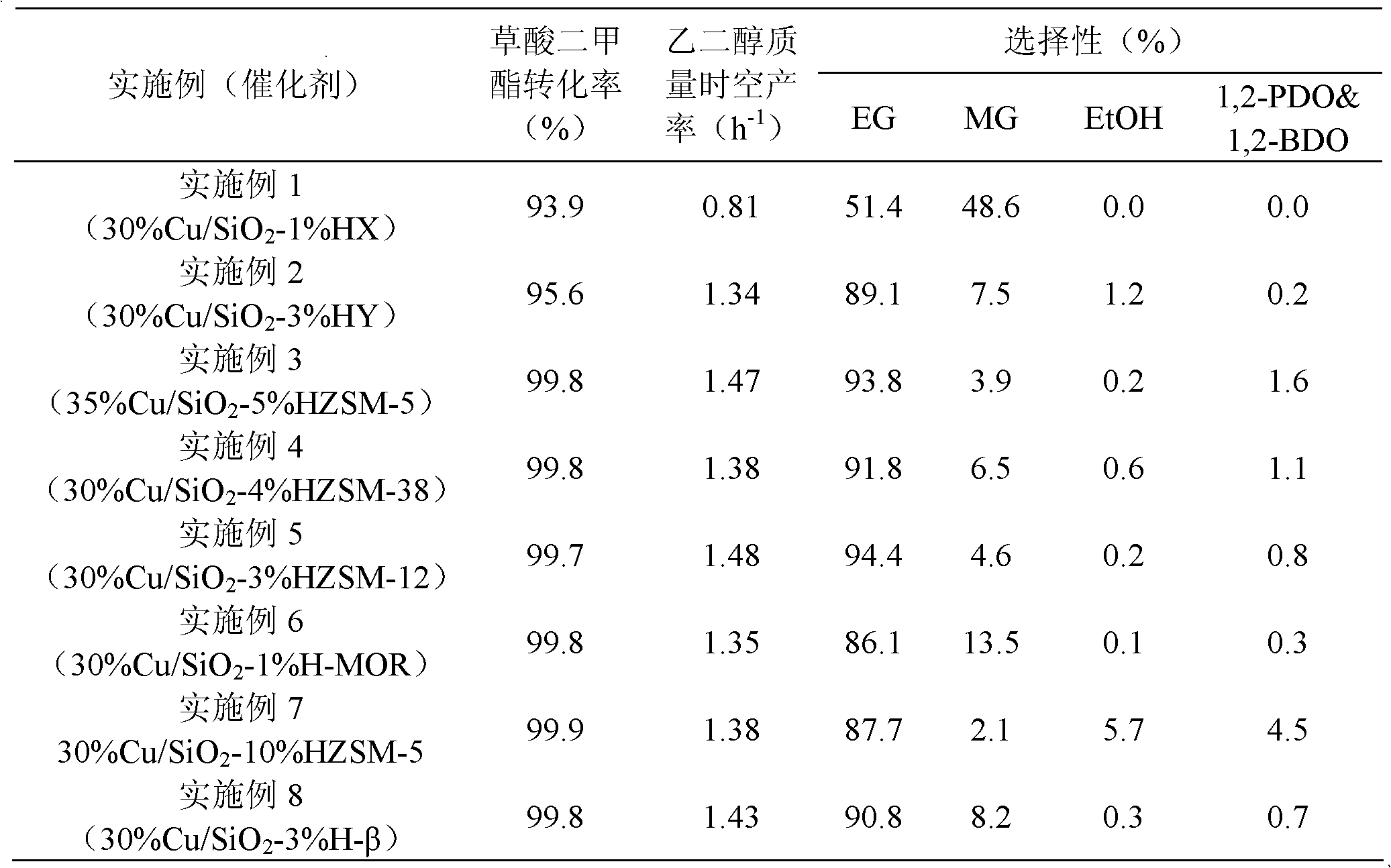
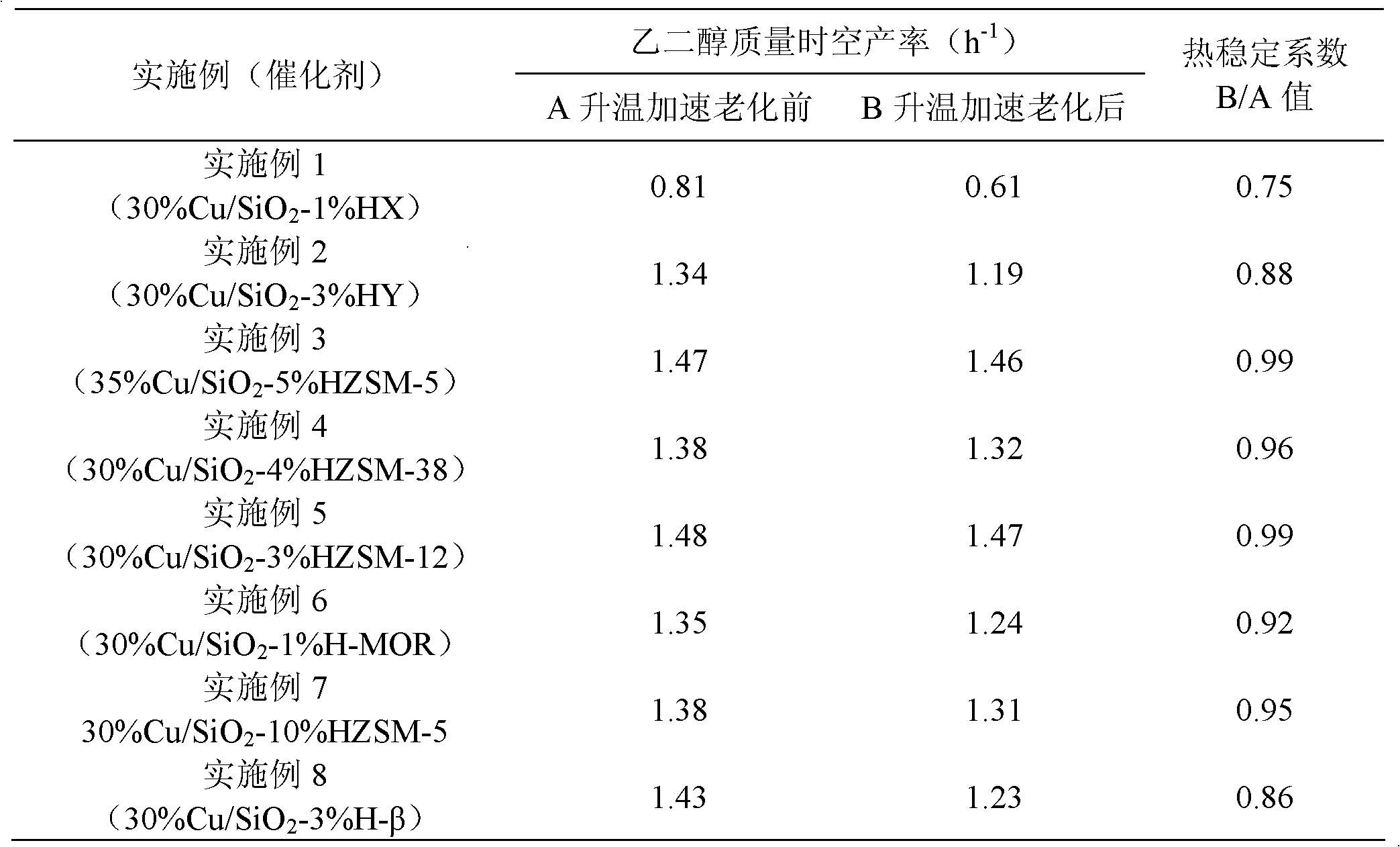
Embodiment 1
[0023] Weigh 6.84g of copper nitrate trihydrate, dissolve it in 100mL of deionized water, add 28wt% ammonia solution dropwise under stirring until the precipitate disappears, and obtain a transparent dark blue solution, which is transferred into a glass with 0.06g of HX molecular sieve In the container, in the state of strong mechanical stirring, slowly drop 10.35g of 40wt% silica sol and 20ml of 20wt% urea aqueous solution at a speed of about 1ml / min successively, raise the temperature to 60°C and stir at a speed of 500r / min for 8h, after cooling, After the precipitate was washed to neutral, it was dried at 120°C for 12 hours, placed in a muffle furnace at a rate of 4°C / min to 350°C, and calcined for 2 hours to obtain a catalyst precursor. Press the tablet and sieve out 40~60 mesh particles, put the catalyst into the reactor, under 5% H 2 30% Cu / SiO was obtained by reducing the temperature at 2°C / min to 350°C for 4 hours under / Ar atmosphere 2 -1% HX catalyst, ICP-MS quantit...
Embodiment 2
[0027] Weigh 6.84g copper nitrate trihydrate, dissolve it in 100mL deionized water, add 28wt% ammonia solution dropwise under stirring until the blue precipitate disappears, transfer it into a glass container equipped with 0.16g HY molecular sieve, and stir it vigorously 10.20g of 40wt% silicate and 30ml of 20wt% urea aqueous solution were slowly dropped at a speed of about 1ml / min, and the temperature of the water bath was raised to 70°C and stirred at a speed of 400r / min for 8h. After cooling, the precipitate After suction filtration and washing to neutrality, dry at 120°C for 12h, place in a muffle furnace to raise the temperature to 450°C at a rate of 4°C / min, and calcinate for 4h to obtain a catalyst precursor. Press the tablet and sieve out 40~60 mesh particles, put the catalyst into the reactor, under 5% H 2 30% Cu / SiO was obtained after reduction at 1°C / min to 320°C for 4 hours under Ar atmosphere 2 -3% HY catalyst, ICP-MS analysis results show that the copper content...
Embodiment 3
[0030] Weigh 6.59g copper acetate monohydrate, dissolve it in 120mL deionized water, add 28wt% ammonia solution dropwise under stirring until the blue precipitate disappears, and transfer it into ) in a glass container under strong mechanical stirring, slowly drop 9.47g of 40wt% silica sol and 30ml of 20wt% urea aqueous solution successively at a speed of about 1ml / min, and raise the temperature of the water bath to 70°C at a speed of 400r / min After stirring for 6 hours, after cooling, the precipitate was filtered and washed until neutral, dried at 120°C for 12 hours, placed in a muffle furnace at a rate of 4°C / min to 450°C, and calcined for 4 hours to obtain a catalyst precursor. Press the tablet and sieve out 40~60 mesh particles, put the catalyst into the reactor, under 5% H 2 35% Cu / SiO was obtained after reduction at 1°C / min to 320°C for 4 hours under Ar atmosphere 2 -5% HZSM-5 catalyst, ICP-MS analysis results show that the copper content is 32.7wt%; after nitrogen stat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com