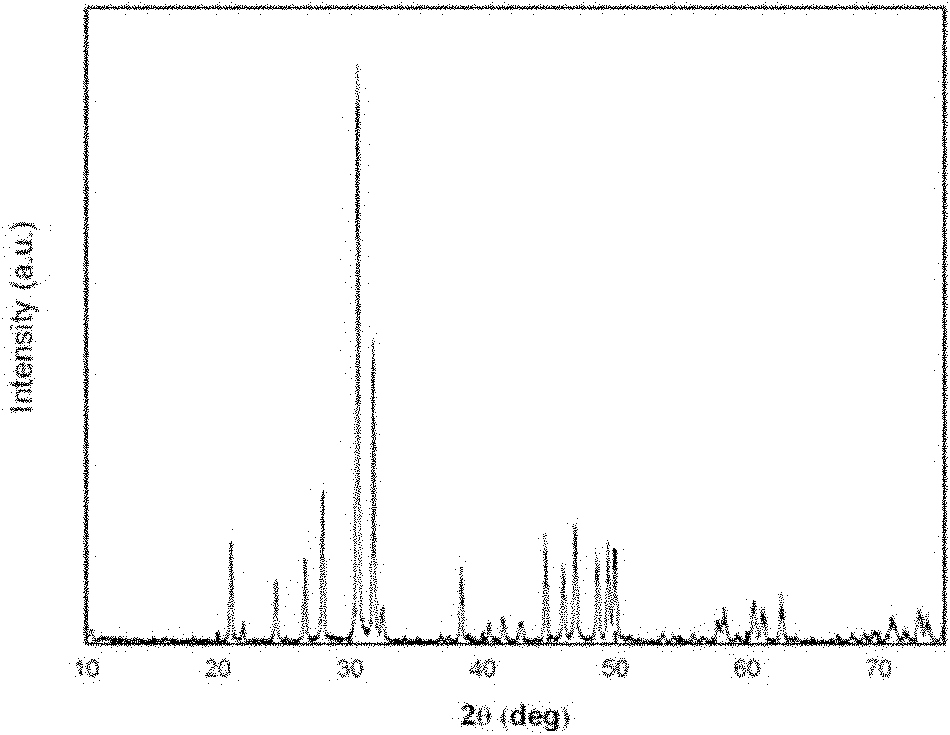
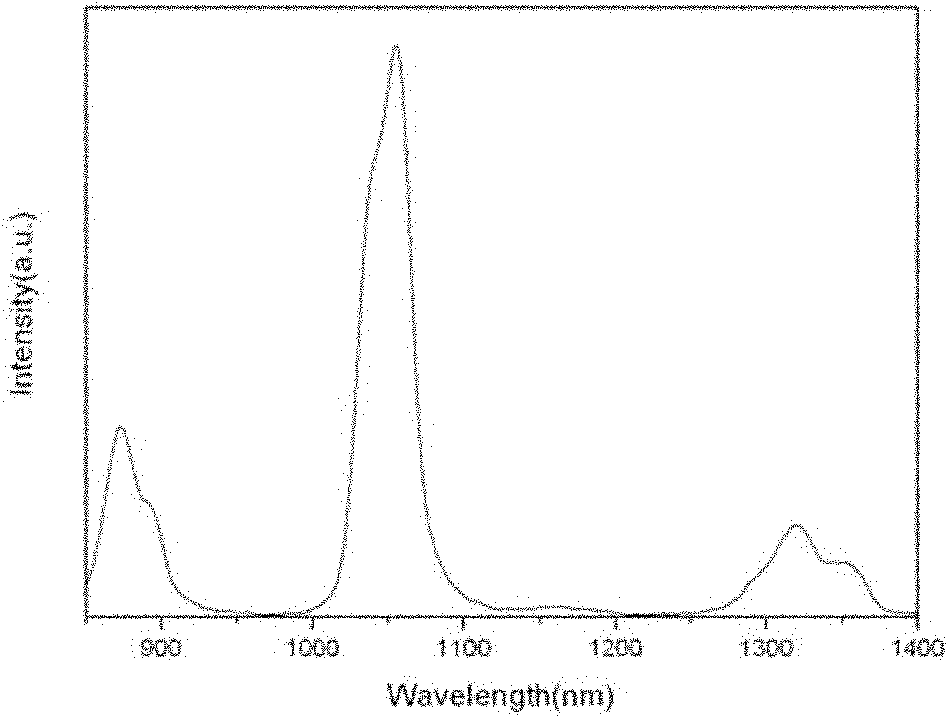
Preparation method of rare-earth-doped strontium fluorophosphate nanoparticles
A nanoparticle, strontium fluorophosphate technology, applied in the field of optical materials, can solve the problems of rare earth ion fluorescence quenching, reduction of fluorescence efficiency, limitation, etc., and achieve the effect of good fluorescence intensity and fluorescence lifetime
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation method of the rare earth-doped strontium fluorophosphate nanoparticles comprises the following steps:
[0025] 1] Mix the rare earth compound or oxide with the aqueous solution of strontium salt, and mix evenly with a magnetic stirrer; then, add the aqueous solution of monohydrogen phosphate and fluoride, and stir for 30 minutes.
[0026] The molar ratio of strontium salt to monohydrogen phosphate is 5:3, the molar ratio of strontium ion to fluoride ion is 5:1-3; the concentration of rare earth ion used is 0.1-1mol / L, the molar ratio of rare earth ion to strontium ion The ratio is 0.005~0.05:1;
[0027] Commonly used rare earth ions are praseodymium, neodymium, samarium, europium, holmium, erbium, thulium, ytterbium, etc., which can be provided by soluble salts of these rare earth ions, such as nitrate, or prepared as a solution with their oxides and nitric acid. The fluoride can be sodium fluoride, ammonium fluoride, etc.; strontium salt is a soluble s...
Embodiment 1
[0033] Weigh 1.047g Sr(NO 3 ) 2 Dissolve in 15ml deionized water, add 0.1ml, 0.25mol / L Pr(NO 3 ) 3 solution (the doping molar ratio is 0.5%) and mix uniformly; weigh 0.396g (NH 4 ) 2 HPO 4 Dissolve 0.042g NaF in 10ml deionized water; mix the above two solutions, and stir for 30 minutes; adjust the pH value of the solution to 3 with 0.01mol / L dilute nitric acid solution, and stir for 1 hour; then, use 0.01mol / L hydrogen The sodium oxide solution adjusted the pH value of the solution to 7, and stirred for 1 hour; the reciprocating cycle was repeated 3 times. The resulting precipitate was centrifuged, washed three times with deionized water and absolute ethanol, and dried in vacuum at 200°C for 5 hours to obtain a white powder.
Embodiment 2
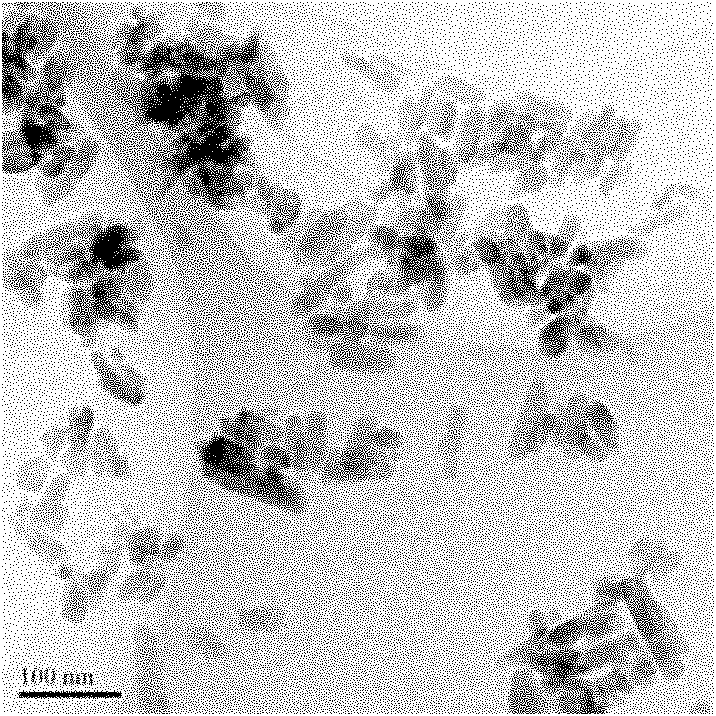
[0035] Weigh 1.047g Sr(NO 3 ) 2 Dissolve in 15ml deionized water, add 0.5ml, 0.1mol / L Nd(NO 3 ) 3 solution (1% molar ratio doping) and mix uniformly; weigh 0.396g (NH 4 ) 2 HPO 4 Dissolve 0.084g NaF in 10ml deionized water, mix the above two solutions, stir for 30 minutes, adjust the pH value of the solution to 3 with 0.1mol / L dilute nitric acid solution, stir for 1 hour, and then add 0.1mol / L The pH value of the solution was adjusted to 7 with sodium hydroxide solution, stirred for 1 hour, and reciprocated 5 times. The obtained precipitate was separated by centrifugation, washed three times with deionized water and absolute ethanol respectively, and dried under vacuum at 70° C. for 24 hours to obtain a white powder. figure 1 Sr prepared for this example5 (PO 4 ) 3 F:Nd 3+ (1% mol) transmission electron microscope image (TEM) of nanoparticle, shows that the morphology of nanoparticle is basically rod-shaped, with a length of about 50nm and a width of about 7nm, and ha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com