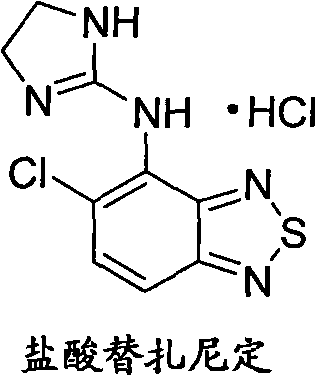
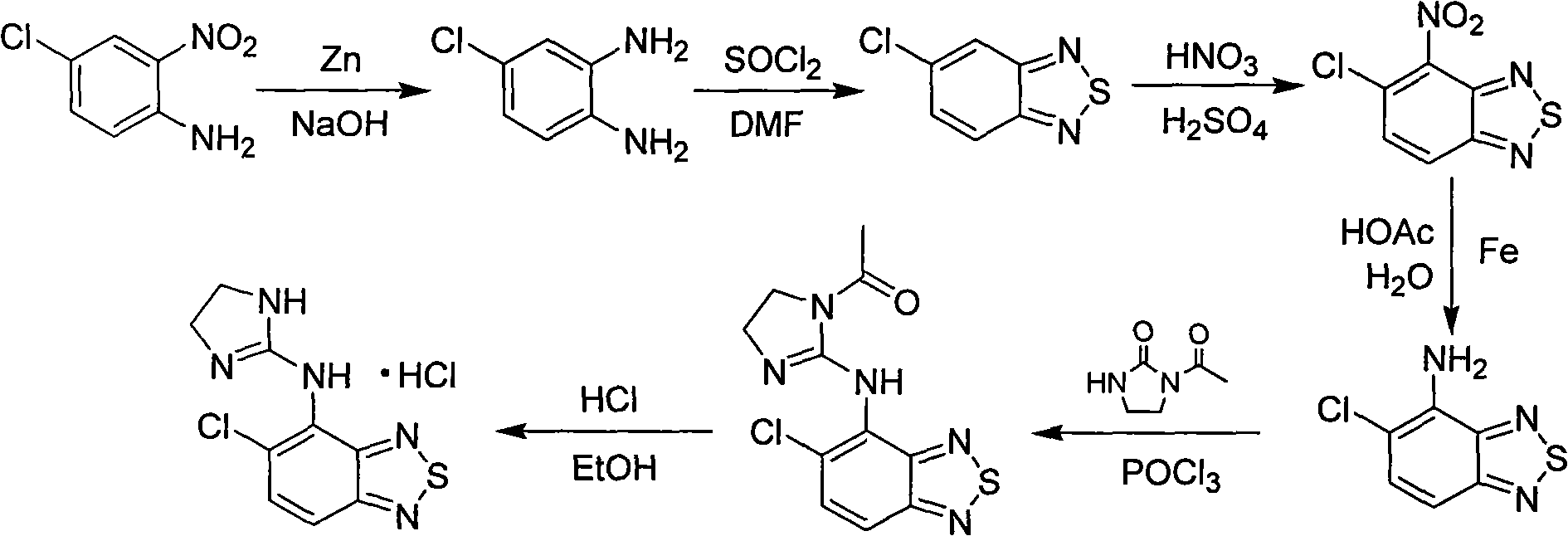
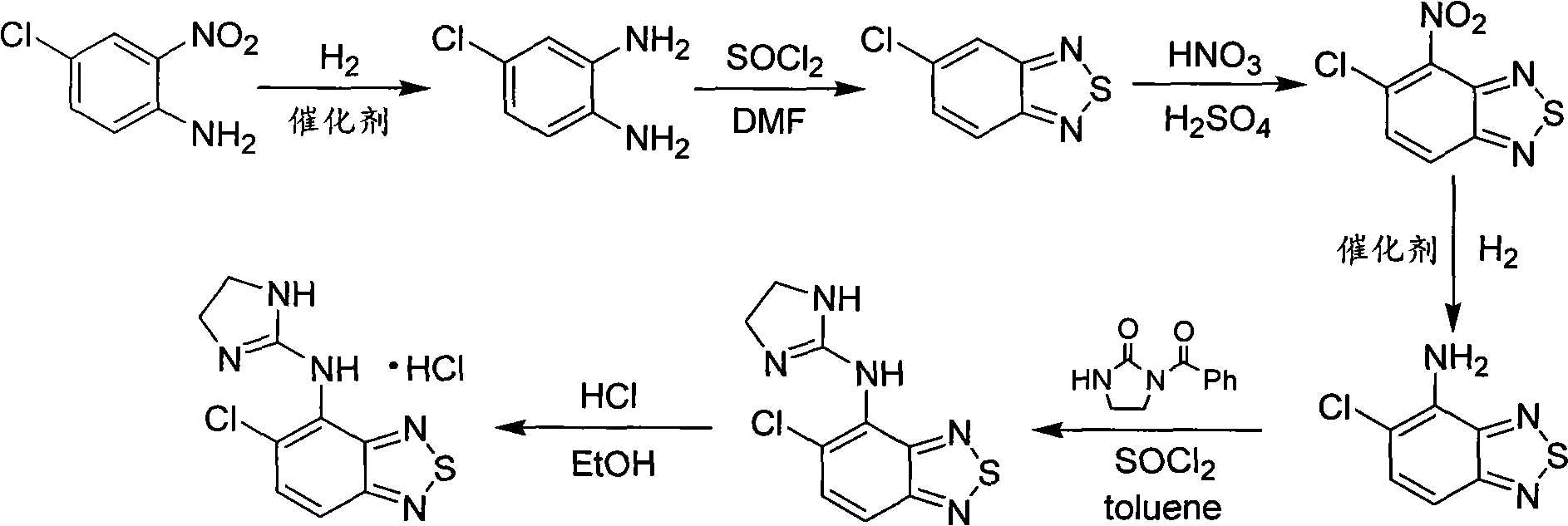
Green new process for preparing tizanidine hydrochloride
A technology of tizanidine hydrochloride and new process is applied in the field of new process for preparing tizanidine hydrochloride, a pharmaceutical raw material, and can solve the problems of strong corrosion of equipment, complicated handling, environmental hazards and the like, and achieves reduction of production cost and reduced The effect of pollution and dosage reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of 4-chloro o-phenylenediamine
[0023] Add 5L of ethanol and 2.5Kg of 4-chloro-2-nitroaniline into a 10L hydrogenation reactor, add 125g of 5% palladium carbon, then pass hydrogen three times to replace the air in the reactor, and heat up to 65-70 °C, keep the pressure inside the kettle at 2Mpa. After the reaction was complete as detected by TLC, the palladium carbon was filtered off, and the filter cake was washed twice with ethanol. The filtrate and washings were combined and concentrated to dryness under reduced pressure to obtain 1.88 Kg of 4-chloro-o-phenylenediamine with a yield of 91%.
Embodiment 2
[0024] Embodiment 2: the synthesis of 4-chloro o-phenylenediamine
[0025] Add 5L of ethanol and 2.5Kg of 4-chloro-2-nitroaniline into a 10L hydrogenation reactor, add 250g of Raney-Ni, then pass hydrogen three times to replace the air in the reactor, and raise the temperature to 65-70°C. Keep the pressure in the kettle at 2Mpa. After the reaction was detected by TLC, the Raney-Ni was filtered off, and the filter cake was washed twice with ethanol. The filtrate and washings were combined and concentrated to dryness under reduced pressure to obtain 1.96 Kg of 4-chloro-o-phenylenediamine with a yield of 95%.
Embodiment 3
[0026] Embodiment 3: the synthesis of 4-chloro o-phenylenediamine
[0027] Add 5L of methanol and 2.5Kg of 4-chloro-2-nitroaniline into a 10L hydrogenation reactor, add 125g of platinum oxide, then pass hydrogen three times to replace the air in the reactor, raise the temperature to 60-65°C, and keep The pressure in the kettle is 3.0Mpa. After the reaction was detected by TLC, the Raney-Ni was filtered off, and the filter cake was washed twice with ethanol. The filtrate and washings were combined and concentrated to dryness under reduced pressure to obtain 1.86 Kg of 4-chloro-o-phenylenediamine with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com