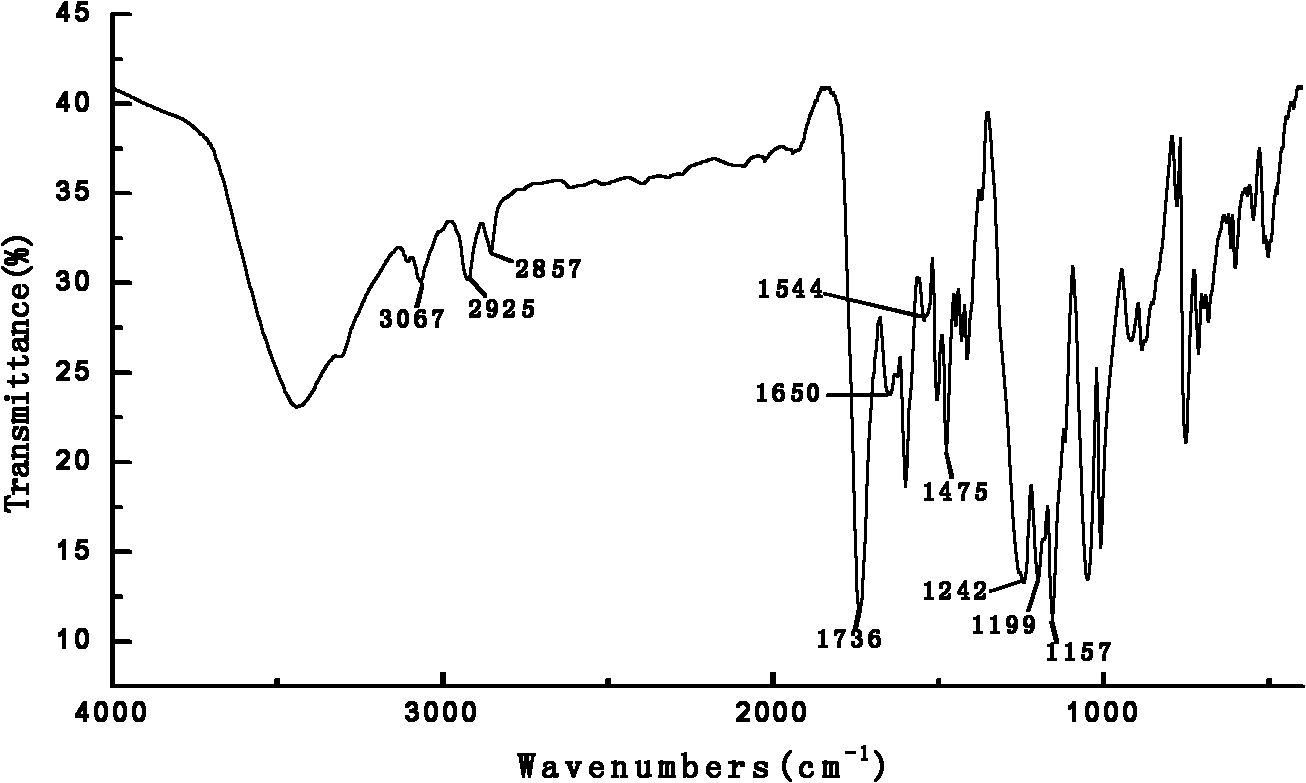
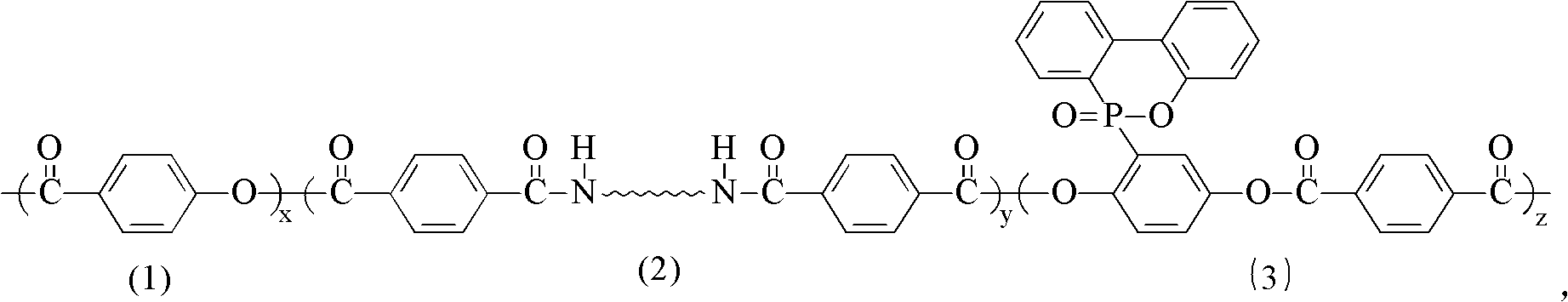
Novel phosphorus and nitrogen containing flame-retardant thermotropic liquid crystal copolyester with low melting point and synthesis method thereof
A technology of thermotropic liquid crystal and low melting point, which is applied in the field of chemical synthesis, can solve problems such as the complex structure and reduction of the flexible unit imide diacid that are not conducive to the melting point of TLCP, and achieve high yield, good reproducibility, and synthetic The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 0.013mol p-acetoxybenzoic acid (p-AHB), 0.004mol 10-(2,5-dihydroxyphenyl)-10-hydrogen-9-oxa10-phosphaphenanthrene-10-oxide Diacetate (ODOPB), 0.003mol terephthalic acid (TPA) and the condensation product of 0.002mol terephthalic acid and 1,6-hexanediamine. The reaction raw materials were charged into a three-necked flask, and a nitrogen device was installed. Repeatedly pass nitrogen and vacuumize to discharge the oxygen in the three-necked bottle. The oil bath was heated to 170°C, and the reaction was carried out under strong stirring and nitrogen protection, and the temperature was raised at a heating rate of 1°C / 2min. When the temperature rose to 250°C, the reaction system showed obvious agitation opalescence, the viscosity was high, and the small molecule by-product acetic acid was not easy to escape. At this time, the nitrogen flow was stopped and the oil pump was used to evacuate. Continue to heat up to 270°C, the pressure in the reaction bottle remains bas...
Embodiment 2
[0035] Weigh 0.015 mol of p-AHB, 0.005 mol of ODOPB, 0.002 mol of TPA and 0.002 mol of the condensation product of terephthalic acid and 1,6-hexanediamine. The reaction raw materials were charged into a three-necked flask, and a nitrogen device was installed. Repeatedly pass nitrogen and vacuumize to discharge the oxygen in the three-necked bottle. The oil bath was heated to 165°C, and the reaction was carried out under strong stirring and nitrogen protection, and the temperature was raised at a heating rate of 1°C / 2min. When the temperature rose to 243°C, the reaction system showed obvious agitation opalescence, the viscosity was high, and the small molecule by-product acetic acid was not easy to escape. At this time, the nitrogen flow was stopped and an oil pump was used to evacuate. Continue to heat up to 268 ° C, the pressure in the reaction bottle remains basically unchanged, indicating that small molecules of acetic acid are no longer generated. After the reaction was ...
Embodiment 3
[0037]Weigh 0.013 mol of p-AHB, 0.004 mol of ODOPB, 0.003 mol of TPA and 0.002 mol of the condensation product of terephthalic acid and 1,7-heptanediamine. The reaction raw materials were charged into a three-necked flask, and a nitrogen device was installed. Repeatedly pass nitrogen and vacuumize to discharge the oxygen in the three-necked bottle. The oil bath was heated to 172°C, and the reaction was carried out under strong stirring and nitrogen protection, and the temperature was raised at a heating rate of 1°C / 2min. When the temperature rose to 248°C, the reaction system showed obvious stirring opalescence, the viscosity was high, and the small molecule by-product acetic acid was not easy to escape. At this time, the nitrogen flow was stopped and the oil pump was used to evacuate. Continue to heat up to 275 ° C, the pressure in the reaction bottle remains basically unchanged, indicating that small molecules of acetic acid are no longer generated. After the reaction was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com