Gallium nitride/zinc oxide solid solution with high zinc content and preparation method thereof
A technology of zinc oxide and gallium nitride, which is applied in the field of gallium nitride/zinc oxide solid solution with high zinc content, can solve the problems of low content and achieve the effects of high production yield, simple preparation method and great industrialization value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
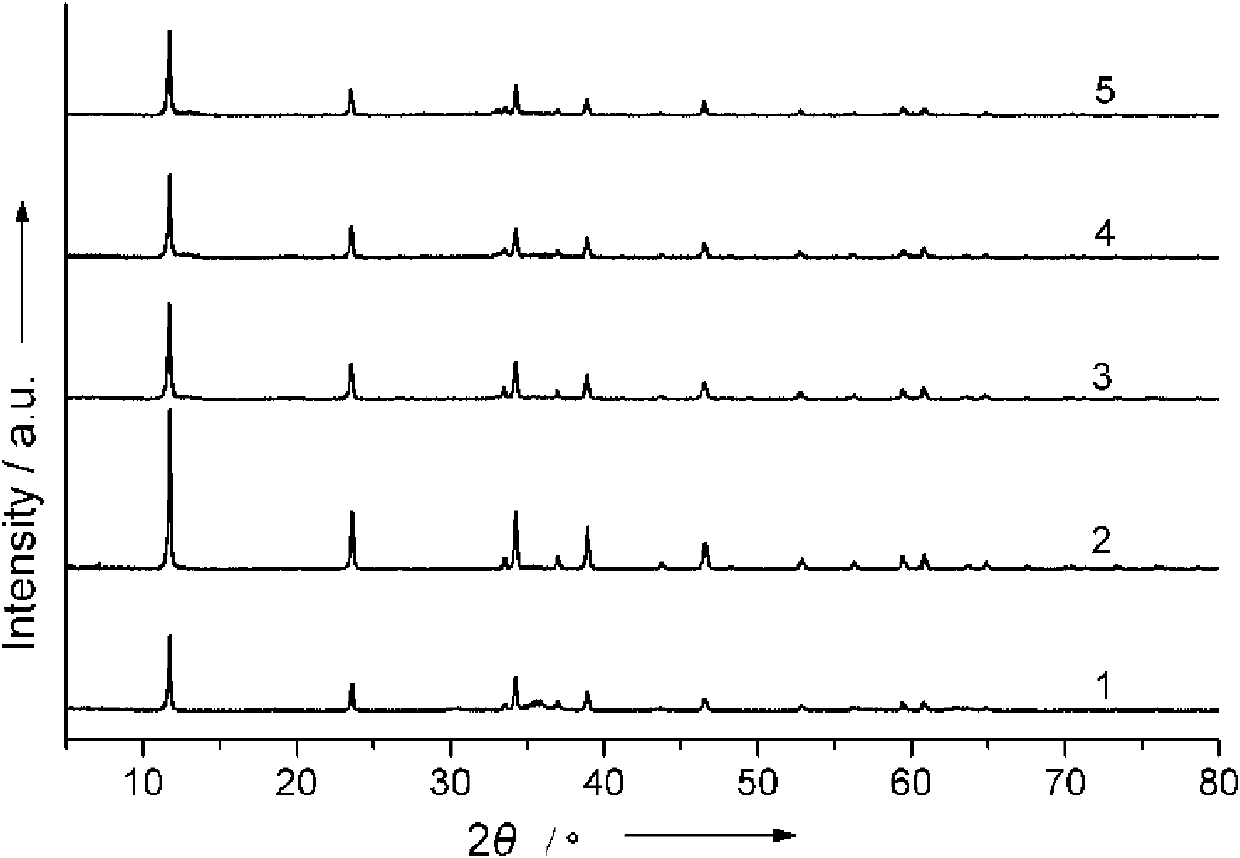
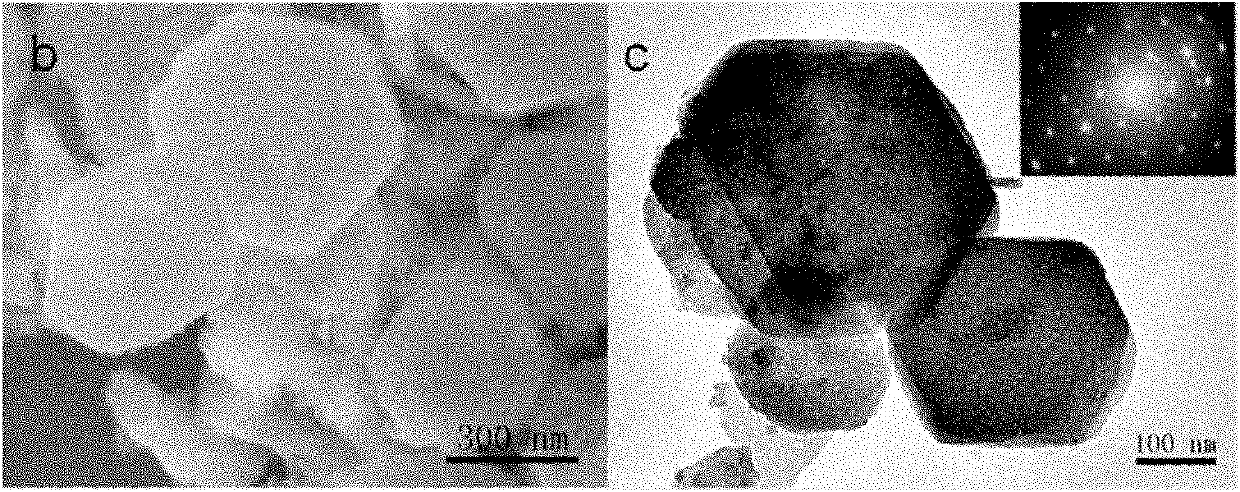
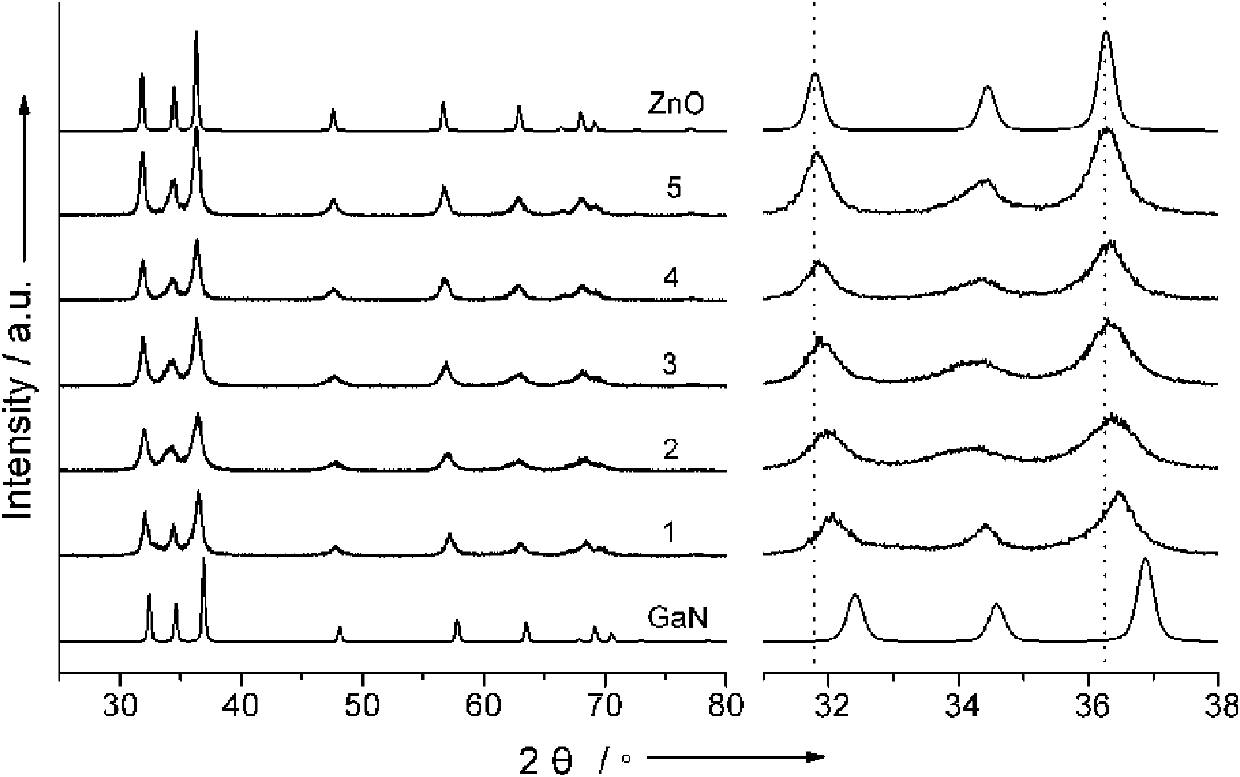
Image
Examples
Embodiment 1
[0026] First, Zn / Ga / CO was synthesized by co-precipitation method 3 The double metal hydroxide precursor: dissolve 0.4g gallium oxide in 10ml boiling concentrated nitric acid (6M), add 0.35g zinc oxide after natural cooling, after dissolving, under vigorous stirring, use sodium hydroxide / sodium carbonate The pH value of the above acid solution was adjusted to 8 with the mixed solution to obtain a white suspension. The white suspension was put into an oven and kept at 80°C for 24h. Subsequently, the white precipitate was washed with deionized water and suction-filtered, and then vacuum-dried at 50° C. for 4 h. That is, Zn / Ga / CO is obtained 3 (Zn:Ga=1) double metal hydroxide.
[0027] Take 0.5g of the resulting Zn / Ga / CO 3 The double metal hydroxide was calcined at 800°C for 30min under an ammonia atmosphere. (Ammonia gas flow rate is controlled at 300ml / min) GaN / ZnO solid solution powder is obtained after natural cooling to room temperature.
Embodiment 2
[0029] Dissolve 0.4g of gallium oxide in 10ml of boiling concentrated nitric acid (6M), add 0.69g of zinc oxide after natural cooling, after dissolving, adjust the pH value of the above acid solution with a mixed solution of sodium hydroxide / sodium carbonate under vigorous stirring To 8, a white suspension was obtained. The white suspension was put into an oven and kept at 80°C for 24h. Subsequently, the white precipitate was washed with deionized water and suction-filtered, and then vacuum-dried at 50° C. for 4 h. That is, Zn / Ga / CO is obtained 3 (Zn:Ga=2) double metal hydroxide.
[0030] Take 0.5g of the resulting Zn / Ga / CO 3 The double metal hydroxide was calcined at 800°C for 30min under an ammonia atmosphere. (Ammonia gas flow rate is controlled at 300ml / min) After naturally cooling to room temperature, yellow gallium nitride / zinc oxide solid solution powder is obtained.
Embodiment 3
[0032] Dissolve 0.4g of gallium oxide in 10ml of boiling concentrated nitric acid (6M), add 1.05g of zinc oxide after natural cooling, after dissolving, adjust the pH value of the above acid solution with a mixed solution of sodium hydroxide / sodium carbonate under vigorous stirring To 8, a white suspension was obtained. The white suspension was put into an oven and kept at 80°C for 24h. Subsequently, the white precipitate was washed with deionized water and suction-filtered, and then vacuum-dried at 50° C. for 4 h. That is, Zn / Ga / CO is obtained 3 (Zn:Ga=3) double metal hydroxide.
[0033] Take 0.5g of the resulting Zn / Ga / CO 3 The double metal hydroxide was calcined at 800°C for 30min under an ammonia atmosphere. (Ammonia gas flow rate is controlled at 300ml / min) After naturally cooling to room temperature, yellow gallium nitride / zinc oxide solid solution powder is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com