D-fructopyranose derived saccharide beta-alkamine and synthesis method thereof
A synthesis method and technology of amino alcohols, applied in sugar derivatives, sugar derivatives, preparation of sugar derivatives, etc., to achieve the effects of cheap and easy-to-obtain raw materials, mild reaction conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
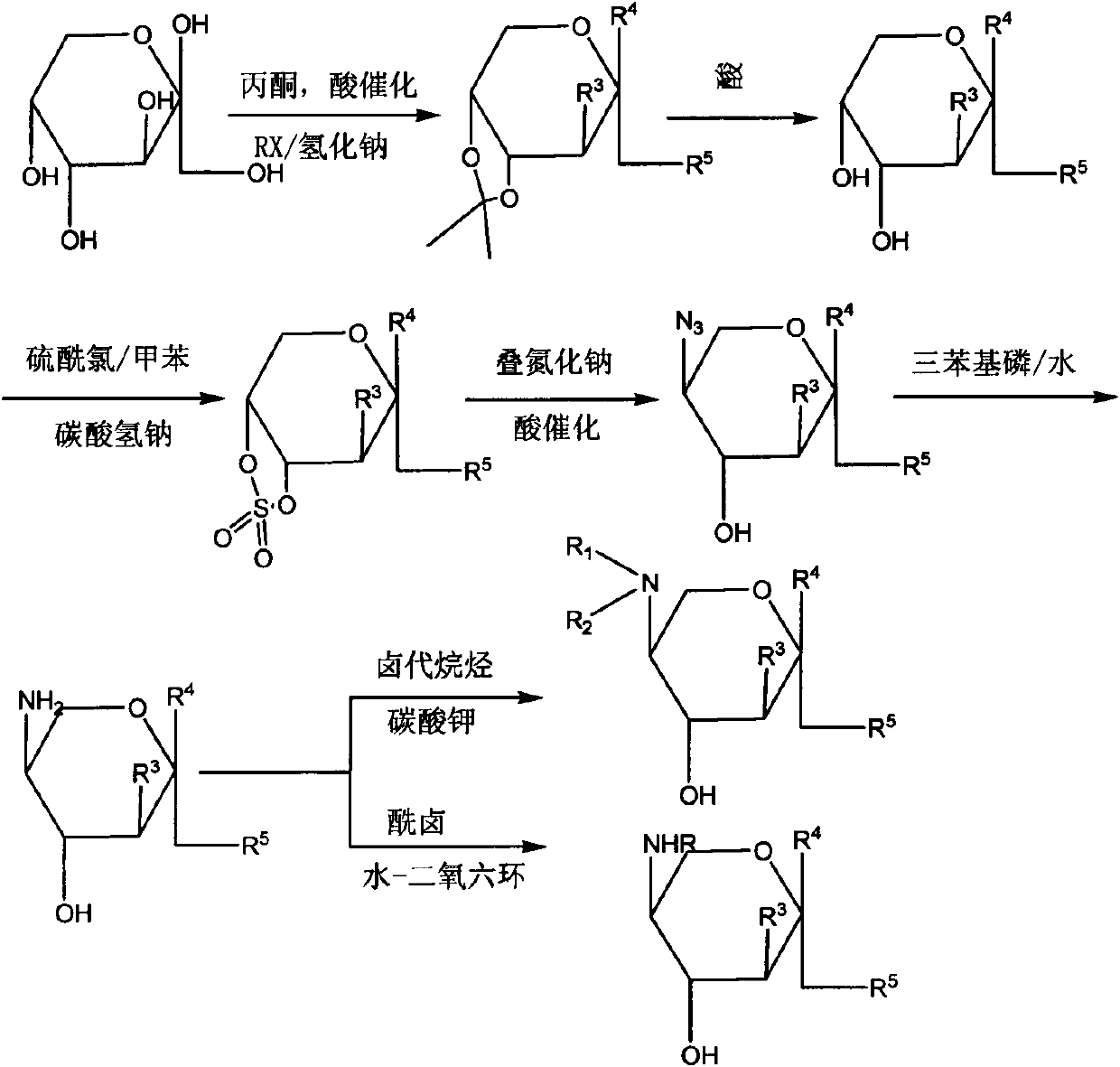
[0029] combine figure 1 , the synthetic method of the carbohydrate β-amino alcohol derived from D-fructose of the present invention, the steps are as follows:
[0030] Step 1: Preparation of 3-O-benzyl-1,2:4,5-bis-O-(1-methyl)ethylene-β-D-fructopyranose, that is, this part includes two steps:
[0031] The first step, the preparation of 1,2:4,5-bis-O-(1-methyl)ethylene-β-D-fructopyranose, various monosaccharides have been catalyzed by acid in this field, Hydroxyl protection to generate diacetonylidene (herein acetonylidene refers to methylene dioxy) technology, including concentrated sulfuric acid catalysis, phosphoric acid catalysis, iodine catalysis, concentrated sulfuric acid catalysis is used in this field, that is, at room temperature, in acetone solution Add concentrated sulfuric acid dropwise. When the amount of concentrated sulfuric acid relative to D-fructose is 0.3-0.4 equivalent, then add D-fructose to the system. After the reaction, add sodium hydroxide solution an...
Embodiment 1
[0053] 1,2: Preparation of 4,5-bis-O-(1-methyl)ethylene-β-D-fructopyranose 1
[0054]
[0055] Add 600mL of acetone to a 1L four-neck flask, add 3mL (0.0553mol) of concentrated sulfuric acid dropwise at room temperature, add 30.6g (0.167mol) of D-fructose at one time, stir at room temperature for 2 hours, and drop 84mL of NaOH solution at 0°C to end the reaction. When the system was light yellow, a large amount of solids were formed. Filtration and concentration gave a yellow solid, 200mLCH 2 Cl 2 , 400mL water wash, water phase with 2 × 200mLCH 2 Cl 2 Extract, combine the organic phases, wash with 2×150mL water, 2×150mL saturated sodium chloride solution successively, Na 2 SO 4 Dry, filter, and concentrate to obtain a light yellow solid, recrystallize from 100 mL petroleum ether, filter, and dry to obtain a white solid 1,2:4,5-bis-O-(1-methyl)ethylene-β-D-pyridine Fructose (67% yield).
Embodiment 2
[0057] 1,2: Preparation of 4,5-bis-O-(1-methyl)ethylene-β-D-fructopyranose 1
[0058] According to the operation of Example 1, the amount of concentrated sulfuric acid was changed to 2.7mL (0.0501mol), and its productive rate was 59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com