Method for preparing (S)-3-hydroxyl-3-(2-thienyl)-propionitrile by microbial transformation
A microbial transformation, thiophene-based technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of material transfer resistance, toxicity, and reduced reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of slant medium: wort juice 10g / L, yeast powder 3g / L, peptone 5g / L, glucose 10g / L, agar 20g / L, natural pH value, solvent is water, sterilized at 121°C for 20min to make slant spare;
[0047] Preparation of seed medium: glucose 30g / L, yeast powder 3g / L, ammonium sulfate 5g / L, anhydrous MgSO 4 0.25g / L, K 2 HPO 4 ·3H 2O 1g / L, KH 2 PO 4 1g / L, natural pH value, solvent is water, sterilized at 121°C for 20min, ready for use;
[0048] The preparation of the fermentation medium is the same as that of the seed medium.
[0049] Slant culture: Saccharomyces cerevisiae CGMCC No.2266 strain was inoculated into the slant medium, cultured at 30° C. for 4 to 6 days, and the slant of the bacteria was obtained.
[0050] Seed culture: use an inoculation needle to take a ring of bacteria from the slant of the bacteria and inoculate it into a 250 mL Erlenmeyer flask containing 100 mL of seed medium, and cultivate it at 30°C and 180 r / min for 24 hours to obtain a seed liqu...
Embodiment 2
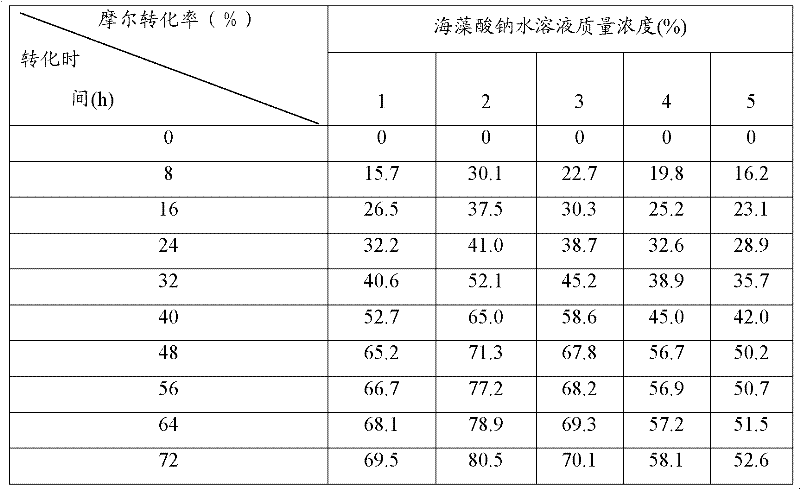
[0057] Saccharomyces cerevisiae CGMCC No.2266 was cultivated according to the method in Example 1 to obtain a bacterial cell fermentation liquid. Mix four parts of 100mL fermentation broth with a cell concentration of 4.3g / L with an equal volume of 2% sodium alginate aqueous solution, and fill the sodium alginate mixture containing bacteria into needles with a size of 2mm, 3mm, and 4mm respectively. and 5mm syringe, drop 500mL 3.5% (w / v) CaCl 2 Form immobilized cell particles in aqueous solution, the diameters of the formed immobilized cell particles are 2mm, 3mm, 4mm and 5mm respectively, solidify at 37°C for 30min, and the obtained immobilized particles are washed with sterile normal saline to remove excess calcium ions and uncaptured cells, the obtained immobilized cell particles were continued to proliferate and culture in 500mL fermentation medium at 30°C for 24h to obtain proliferation cultured immobilized cell particles. The particle size after the proliferation cultur...
Embodiment 3
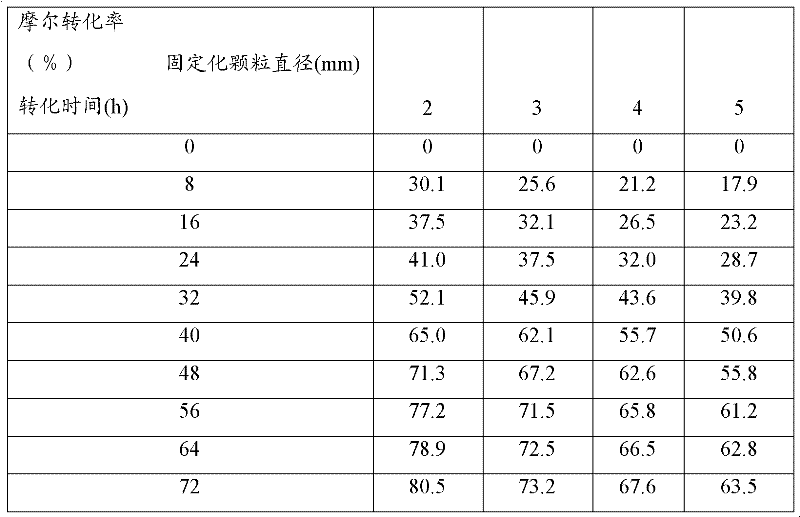
[0062] Saccharomyces cerevisiae CGMCC No.2266 was cultivated according to the method in Example 1 to obtain a bacterial cell fermentation broth. Four parts of 100mL of fermentation broth with a bacterial cell concentration of 4.3g / L were mixed with an equal volume of 2% sodium alginate aqueous solution, and the sodium alginate mixed solution containing the bacterial cell was respectively put into a syringe with a diameter of 2 mm, and dripped into 500mL 3.5% (w / v) CaCl 2 Form immobilized particles in an aqueous solution, the diameter of the formed immobilized particles is 2 mm, solidify at 37 ° C for 30 min, and the obtained immobilized particles are washed with sterile physiological saline to remove excess calcium ions and uncaptured cells, and the obtained 4 The immobilized cell granules were respectively cultured in 500mL fermentation medium at 30°C for 0h, 24h, 48h and 72h to obtain immobilized cell granules after proliferation.
[0063] Add the above 4 portions of immobi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com