Method for preparing carbon-coated LiFePO4 anode material by using low-temperature solid-phase method
A technology for carbon-coated lithium iron phosphate and positive electrode materials, which is applied in battery electrodes, structural parts, electrical components, etc., can solve problems such as unfavorable continuous production, easy generation of impurity phases, easy product agglomeration, etc., and achieves excellent cycle stability. , Good batch consistency, uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
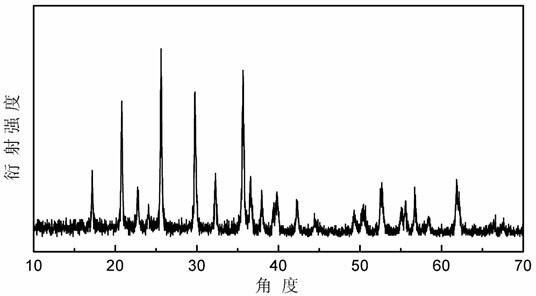
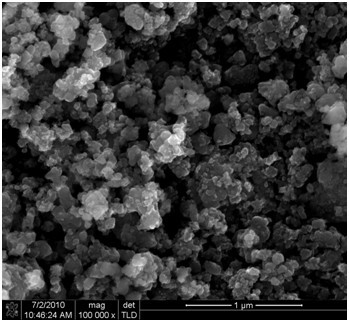
Image
Examples
Embodiment 1
[0050] Weigh iron phosphate and lithium hydroxide according to the molar ratio of iron and lithium as 1:1, weigh stearic acid according to the proportion of 20g stearic acid per mole of iron phosphate, and dissolve the weighed stearic acid in absolute ethanol In, the ethanol solution of the stearic acid that the concentration is 10 g / L is prepared. The ethanol solution of ferric phosphate, lithium hydroxide and stearic acid is mixed and ball milled in a high-energy ball mill for 8 hours, and the slurry precursor obtained after ball milling is stirred in the air to make it evaporate naturally to the mass percentage of absolute ethanol therein 50%. Put the naturally evaporated precursor into a porcelain boat and push it into a tube furnace. Under an argon atmosphere, 400 o Calcined at C for 3 hours, took out the obtained solid after cooling in the furnace, and ground it to obtain a black powder product carbon-coated lithium ferrous phosphate.
[0051] The mass percent content ...
Embodiment 2
[0053] Weigh iron phosphate and lithium hydroxide according to the molar ratio of iron and lithium as 1:1, weigh stearic acid according to the proportion of 20g stearic acid per mole of iron phosphate, and dissolve the weighed stearic acid in absolute ethanol In, the ethanol solution of the stearic acid that the concentration is 10 g / L is prepared. The ethanol solution of ferric phosphate, lithium hydroxide and stearic acid is mixed and ball milled in a high-energy ball mill for 8 hours, and the slurry precursor obtained after ball milling is stirred in the air to make it evaporate naturally to the mass percentage of absolute ethanol therein 60%. Put the naturally evaporated precursor into a porcelain boat and push it into a tube furnace. Under an argon atmosphere, 400 o Calcined at C for 6 hours, cooled with the furnace, took out the obtained solid, and ground it to obtain a black powder product carbon-coated lithium ferrous phosphate.
[0054] The mass percent content of c...
Embodiment 3
[0056] Weigh iron phosphate and lithium hydroxide according to the molar ratio of iron and lithium as 1:1, weigh stearic acid according to the proportion of 20g stearic acid per mole of iron phosphate, and dissolve the weighed stearic acid in absolute ethanol In, the ethanol solution of the stearic acid that the concentration is 10 g / L is prepared. The ethanol solution of ferric phosphate, lithium hydroxide and stearic acid is mixed and ball milled in a high-energy ball mill for 8 hours, and the slurry precursor obtained after ball milling is stirred in the air to make it evaporate naturally to the mass percentage of absolute ethanol therein is 0%. Put the naturally evaporated precursor into a porcelain boat and push it into a tube furnace. Under an argon atmosphere, 400 o Calcined at C for 0.5 hours, cooled with the furnace, took out the obtained solid, and ground it to obtain a black powder product carbon-coated lithium ferrous phosphate.
[0057] The mass percent content ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com