Method for separating polypeptide through hydrogen binding adsorption chromatography of quercitin aglucon and agarose
A technology of hydrogen bond adsorption and chromatographic separation, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of low reusability of reversed-phase chromatography media, poor biocompatibility, and low sample load, etc. Achieve easy scale-up, high recovery, and high sample load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
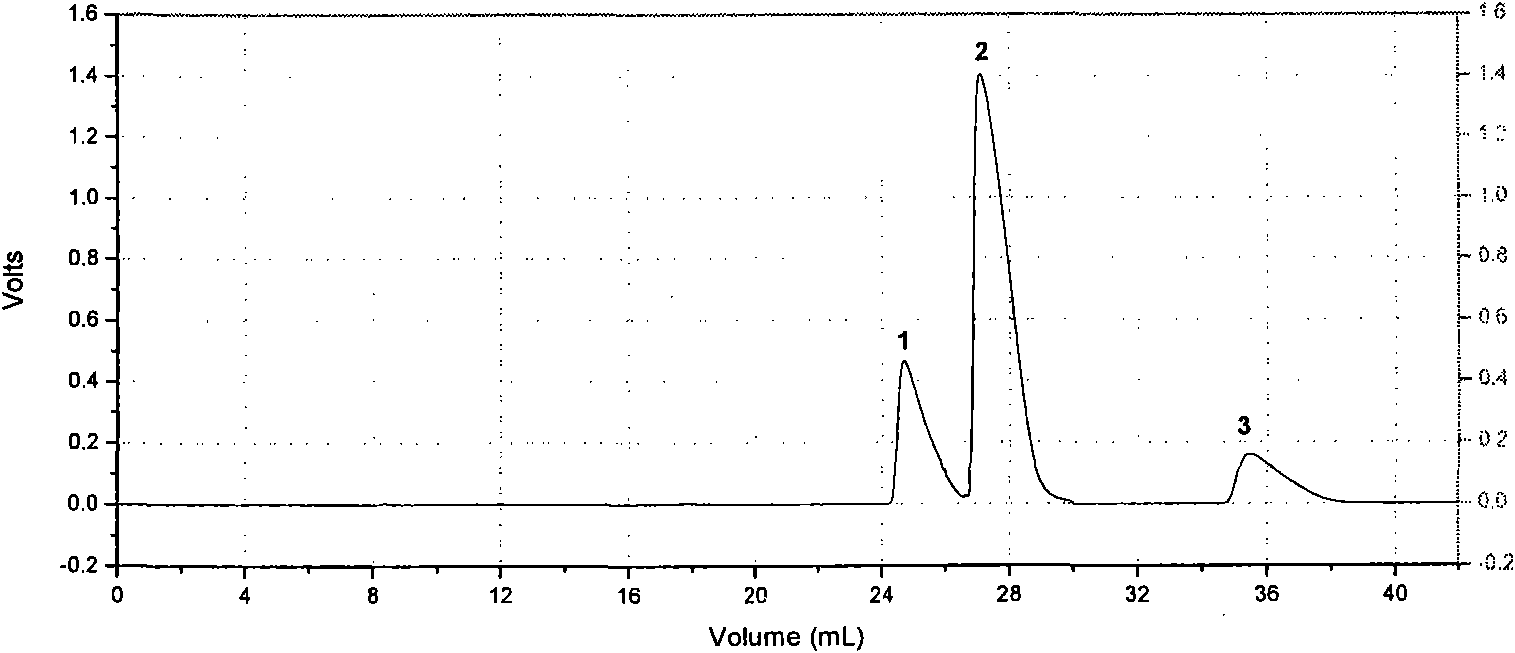
[0030] Example 1. Separation of three glycine oligopeptide mixtures using high cross-linking degree agarose gel hydrogen bond adsorption chromatography medium with 12% concentration of quercetin ligand
[0031]1) Preparation of glycine oligopeptide mixture: Weigh standard products 0.6mgGly-Gly, 1.2mgGly-Gly-Gly and 0.4mgGly-Gly-Gly-Gly-Gly, mix them and dissolve them in 5mL mobile phase, sonicate, 0.45μm Membrane filtration;
[0032] 2) Preparation of mobile phase: preparation of acetonitrile-water (10:90) solution, ultrasonic degassing for 30 minutes, and standing for 1 hour;
[0033] 3) Highly cross-linked agarose gel hydrogen bond adsorption chromatography medium with 12% quercetin ligand is wet-packed in 20% ethanol preservation solution, the inner diameter of the chromatography column is 10mm, the column bed volume is 24mL, and the medium pressure 2.5MPa, the volume of the initial mobile phase equilibrium chromatography column is 2 column beds;
[0034] 4) Inject 500 μL...
Embodiment 2
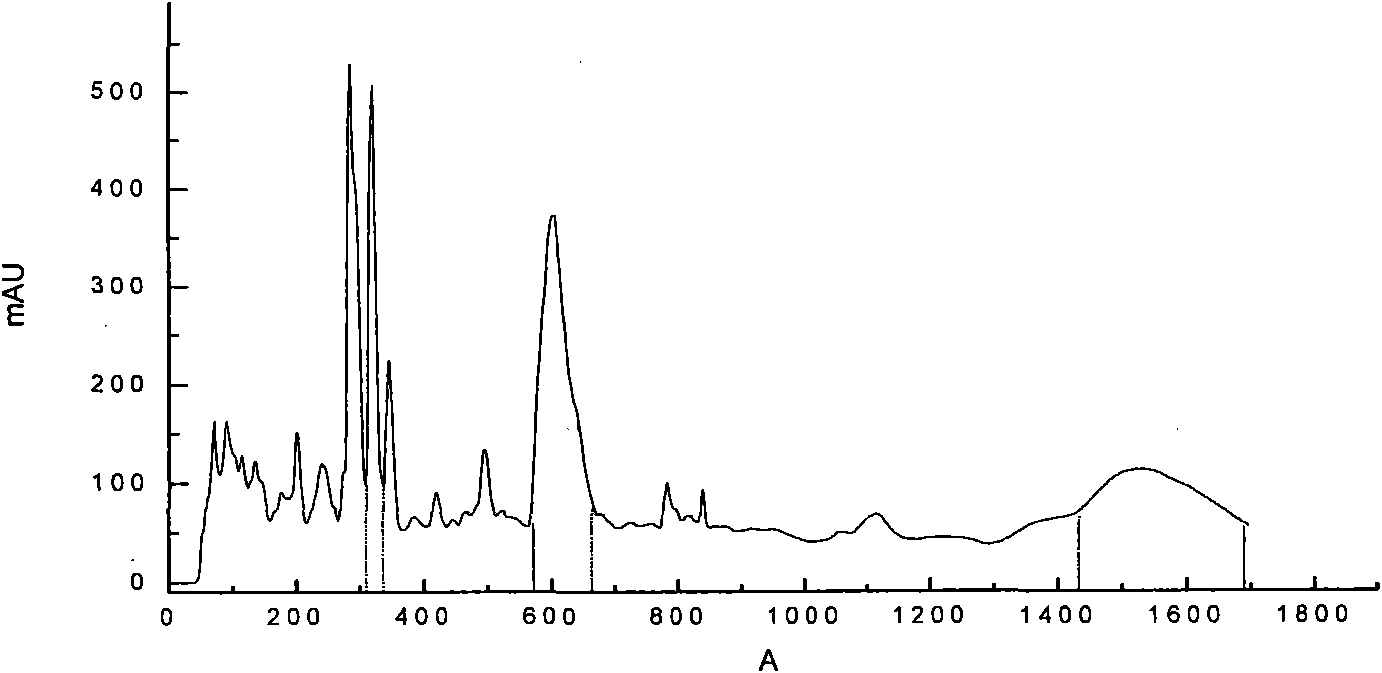
[0037] Example 2. Using quercetin ligand with 12% concentration of high cross-linking degree agarose gel hydrogen bond adsorption chromatography medium to separate and purify liquid phase synthesis of thymopentin mixture
[0038] 1) liquid phase synthesis of thymopentin mixture;
[0039] 2) Preparation of mobile phase: preparation of acetonitrile-water (30:70) solution, ultrasonic degassing for 30 minutes, and standing for 1 hour;
[0040] 3) Highly cross-linked agarose gel hydrogen bond adsorption chromatographic medium with 12% concentration of quercetin ligand, agarose gel medium wet-packed column, the inner diameter of the chromatographic column is 10mm, the column bed volume is 24ml, and the pressure resistance is 2.5MPa. The chromatographic column equilibrates 2 column bed volumes with the initial mobile phase;
[0041] 4) Inject 1ml of the liquid-phase synthetic thymopentin mixture sample through the automatic sampling valve;
[0042] 5) Isocratic elution, generally w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com