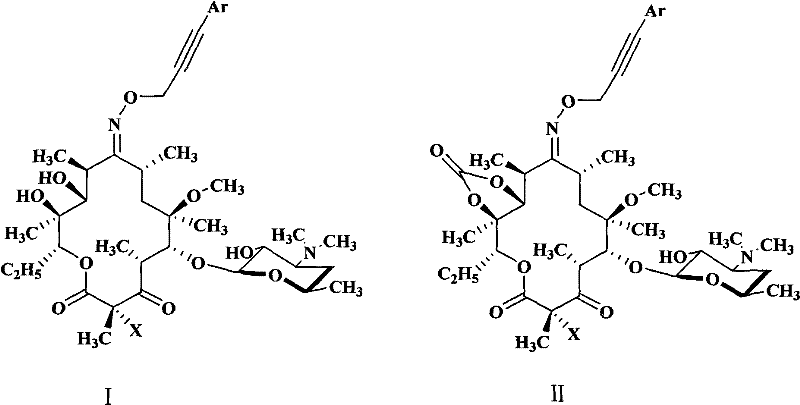
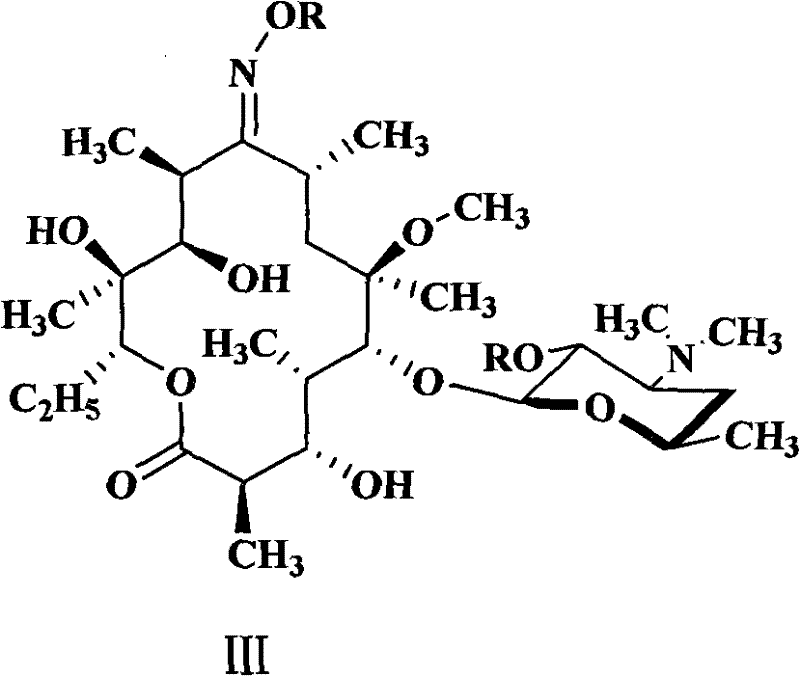
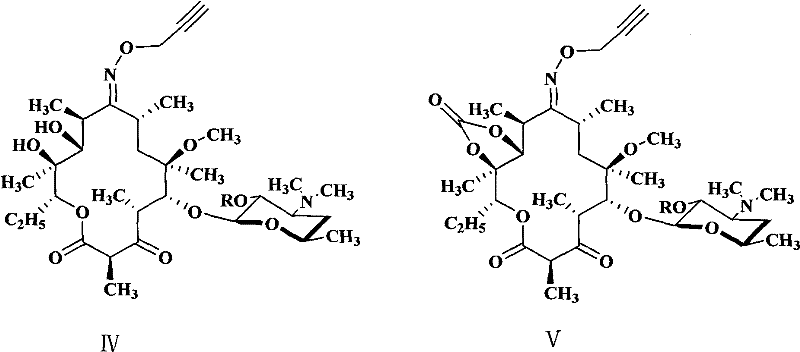
Novel ketolide derivatives, and preparation method and medicinal compositions thereof
A compound and general formula technology, applied in the field of novel ketolactone and its preparation, can solve the problems of less than ideal activity against drug-resistant bacteria, and achieve the effect of simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of the compound with the above general formula is briefly described below.
[0045] The preparation process of the present invention is as follows:
[0046] 1. Starting from erythromycin oxime, the intermediate compound 1,2′,4″-O-bis(trimethylsilyl)-6-O of the clarithromycin synthesis process was obtained through etherification, silanization, and methylation - Methylerythromycin A 9-O-(isopropoxycyclohexyl) oxime;
[0047] 2. Then hydrolyze to obtain the key compound 2: 3-OH-6-O-methylerythromycin oxime;
[0048] 3. Furthermore, under the action of acetic anhydride, 2′-OH and 9-oxime hydroxyl diacetylation to obtain compound 3: 2′-O-acetyl-3-hydroxy-6-O-methylerythromycin A 9 -O-acetyl oxime. The process of the above three steps has been mentioned in the open literature and will not be described in detail here. For similar processes, see Liang Jianhua et al., Organic Chemistry, 2005, 438-441; Zhou Baige et al., Fine Chemical Industry, 2005, 31...
Embodiment 1
[0073] Embodiment 1 (generates compound 2)
[0074] 3-Hydroxy-6-O-methylerythromycin oxime
[0075] Dissolve 13.2g of compound 1 in 40ml of ethanol, dilute 5ml of 36% concentrated HCl into 50ml of water and add dropwise to the reaction solution, react at 40°C for one hour, add ammonia water after the reaction to adjust the pH value to about 9, white precipitates precipitate out , and filter the white precipitate. This precipitate was recrystallized in ethanol and water to obtain 3-OH clarithromycin oxime (5.42g, yield 71%)
Embodiment 2
[0076] Embodiment 2 (generates compound 3)
[0077]2′-O-acetyl-3-hydroxy-6-O-methylerythromycin A 9-O-acetyl oxime
[0078] Add 50ml of dichloromethane into the flask containing 5.42g (8.96mmol) of 3-hydroxy clarithromycin oxime, add dropwise 2.6ml (24.88mmol) of acetic anhydride, and finish the reaction for one hour. , water, washed with saturated brine and filtered, and the solvent was removed under reduced pressure to obtain 5.60 g (8.13 mmol, yield 90.7%) of a white foamy compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com