Diolefin compound, epoxy resin, curable resin composition and cured product
A technology of epoxy resin and curing resin, applied in the direction of organic chemistry, etc., can solve the problems of unoptimized operation, small molecular weight, high cost, etc., and achieve the effect of excellent mechanical properties and low colorability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
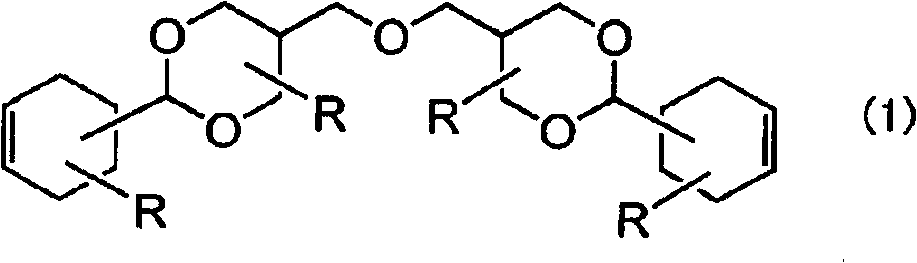
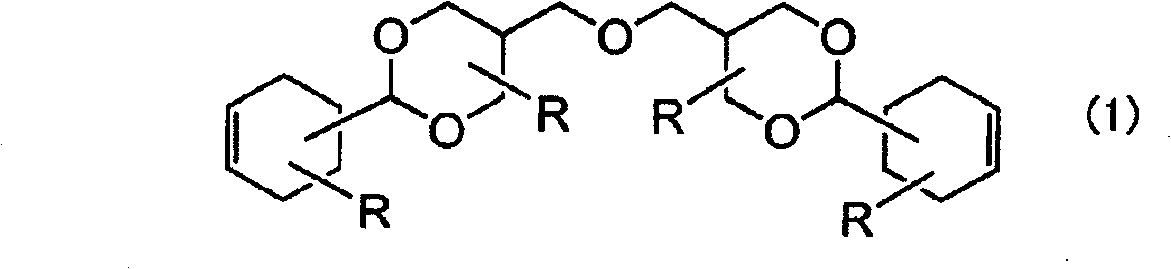
[0134] Into the flask with stirrer, reflux condenser, and stirring device, add 150 parts of water, 55.1 parts of 3-cyclohexene formaldehyde, 62.6 parts of trimethylolpropane, and 7.3 parts of concentrated hydrochloric acid while purging nitrogen gas , and reacted at 60 °C for 10 hours. After completion of the reaction, 100 parts of water and 30 parts of 3% sodium hydroxide aqueous solution were added, and then neutralized with disodium hydrogen phosphate. 200 parts of methyl isobutyl ketone was added thereto, and after washing three times with 100 parts of water, the solvent and the like were removed to obtain the following formula (4)
[0135]
[0136] 101 parts of the diene compound (D-1) of the present invention represented.
[0137] The obtained compound was in a liquid form, and its purity was 94% as measured by GC, and 98% was confirmed as a result of GPC analysis. The viscosity is 21000mPa·s (25°C, E-type viscometer).
Embodiment 2
[0139] In the flask with stirrer, reflux condenser, stirring device, Dean-Stark (Dean-Stark) tube, add 150 parts of toluene, 55.1 parts of 3-cyclohexene formaldehyde, two 62.6 parts of trimethylolpropane and 1.5 parts of p-toluenesulfonic acid were reacted for 10 hours while removing water under reflux conditions. After completion of the reaction, 3 parts of sodium tripolyphosphate was added, stirred at 100° C. for 30 minutes, filtered, 200 parts of toluene and 100 parts of 10% by weight disodium hydrogen phosphate aqueous solution were added, washed with water, and washed with water three times with 100 parts of water. 200 parts of methyl isobutyl ketone was added thereto, washed with 100 parts of water three times, and then the solvent and the like were removed to obtain 108 parts of the diene compound (D-2) of the present invention represented by the above formula (4).
[0140] The obtained compound was in a liquid form, and its purity as measured by GC was 93%, and as a re...
Embodiment 3
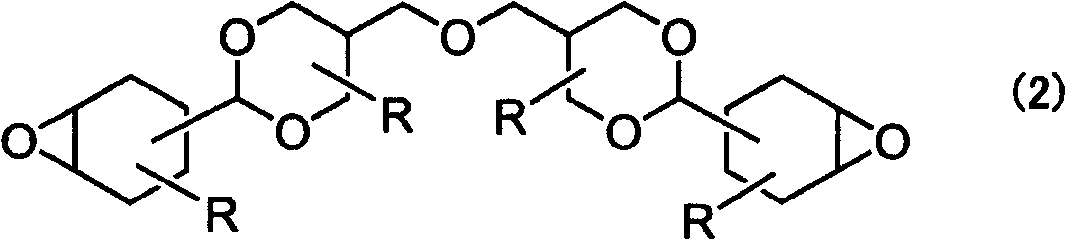
[0142] Add 15 parts of water, 0.95 parts of 12-phosphotungstic acid, 0.78 parts of disodium hydrogen phosphate, dihardened tallow alkyl dimethyl After 2.7 parts of ammonium acetate (Lion Akuke system, 50% by weight hexane solution) and generate tungstic acid series catalyst, add 120 parts of toluene, 108 parts of compound (D-2) of the formula (4) that embodiment 2 obtains, carry out again By stirring, a solution in an emulsion state was obtained. The temperature of this solution was raised to 50 degreeC, 55 parts of 35% hydrogen peroxide aqueous solutions were added, stirring vigorously, and it stirred at 50 degreeC for 13 hours as it was. The progress of the reaction was confirmed by GC. After the reaction, the conversion rate of the substrate was >99%, and the raw material peak disappeared.
[0143] Then, after neutralizing with a 1% aqueous sodium hydroxide solution, 25 parts of a 20% aqueous sodium thiosulfate solution was added, stirred for 30 minutes, and then left to s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com